Regulation of redox signaling by selenoproteins
- PMID: 20306235
- PMCID: PMC2855032
- DOI: 10.1007/s12011-010-8656-7
Regulation of redox signaling by selenoproteins
Abstract
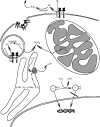
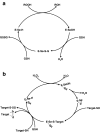
The unique chemistry of oxygen has been both a resource and threat for life on Earth for at least the last 2.4 billion years. Reduction of oxygen to water allows extraction of more metabolic energy from organic fuels than is possible through anaerobic glycolysis. On the other hand, partially reduced oxygen can react indiscriminately with biomolecules to cause genetic damage, disease, and even death. Organisms in all three superkingdoms of life have developed elaborate mechanisms to protect against such oxidative damage and to exploit reactive oxygen species as sensors and signals in myriad processes. The sulfur amino acids, cysteine and methionine, are the main targets of reactive oxygen species in proteins. Oxidative modifications to cysteine and methionine can have profound effects on a protein's activity, structure, stability, and subcellular localization. Non-reversible oxidative modifications (oxidative damage) may contribute to molecular, cellular, and organismal aging and serve as signals for repair, removal, or programmed cell death. Reversible oxidation events can function as transient signals of physiological status, extracellular environment, nutrient availability, metabolic state, cell cycle phase, immune function, or sensory stimuli. Because of its chemical similarity to sulfur and stronger nucleophilicity and acidity, selenium is an extremely efficient catalyst of reactions between sulfur and oxygen. Most of the biological activity of selenium is due to selenoproteins containing selenocysteine, the 21st genetically encoded protein amino acid. The most abundant selenoproteins in mammals are the glutathione peroxidases (five to six genes) that reduce hydrogen peroxide and lipid hydroperoxides at the expense of glutathione and serve to limit the strength and duration of reactive oxygen signals. Thioredoxin reductases (three genes) use nicotinamide adenine dinucleotide phosphate to reduce oxidized thioredoxin and its homologs, which regulate a plethora of redox signaling events. Methionine sulfoxide reductase B1 reduces methionine sulfoxide back to methionine using thioredoxin as a reductant. Several selenoproteins in the endoplasmic reticulum are involved in the regulation of protein disulfide formation and unfolded protein response signaling, although their precise biological activities have not been determined. The most widely distributed selenoprotein family in Nature is represented by the highly conserved thioredoxin-like selenoprotein W and its homologs that have not yet been assigned specific biological functions. Recent evidence suggests selenoprotein W and the six other small thioredoxin-like mammalian selenoproteins may serve to transduce hydrogen peroxide signals into regulatory disulfide bonds in specific target proteins.
Figures
References
-
- Fallab S. Reactions with Molecular Oxygen. Angew Chem Int Ed Eng. 1967;6:496–507. doi: 10.1002/anie.196704961. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

