The perpetual movements of anaphase
- PMID: 20306325
- PMCID: PMC11115923
- DOI: 10.1007/s00018-010-0327-5
The perpetual movements of anaphase
Abstract
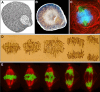
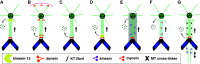
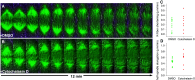
One of the most extraordinary events in the lifetime of a cell is the coordinated separation of sister chromatids during cell division. This is truly the essence of the entire mitotic process and the reason for the most profound morphological changes in cytoskeleton and nuclear organization that a cell may ever experience. It all occurs within a very short time window known as "anaphase", as if the cell had spent the rest of its existence getting ready for this moment in an ultimate act of survival. And there is a good reason for this: no space for mistakes. Problems in the distribution of chromosomes during cell division have been correlated with aneuploidy, a common feature observed in cancers and several birth defects, and the main cause of spontaneous abortion in humans. In this paper, we critically review the mechanisms of anaphase chromosome motion that resisted the scrutiny of more than 100 years of research, as part of a tribute to the pioneering work of Miguel Mota.
Figures


References
-
- Baker J. The cell-theory: a restatement, history, and critique: Part V. The multiplication of nuclei. Q J Microscop Sci. 1955;3–96:449–481.
-
- Kowalevski A. Embryologische Studien an Wurmerm und Arthropoden. Mem Acad Imper Sci St. Petersburg. 1871;16:1–70.
-
- Flemming W. Beiträge zur Kenntniss der Zelle und ihrer Lebenserscheinungen. Arch Mikr Anat. 1879;16:302–436. doi: 10.1007/BF02956386. - DOI
-
- Strasburger E. Die Controversen der indirecten Kerntheilung. Arch Mikr Anat. 1884;23:246–304. doi: 10.1007/BF02952312. - DOI
-
- Carter LA. The somatic mitosis of Stegomyia fasciata . Q J Microscop Sci. 1918;2–63:375–386.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

