Molecular basis of calcium signaling in lymphocytes: STIM and ORAI
- PMID: 20307213
- PMCID: PMC2861828
- DOI: 10.1146/annurev.immunol.021908.132550
Molecular basis of calcium signaling in lymphocytes: STIM and ORAI
Abstract
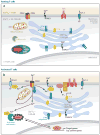
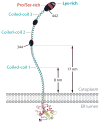
Ca(2+) entry into cells of the peripheral immune system occurs through highly Ca(2+)-selective channels known as CRAC (calcium release-activated calcium) channels. CRAC channels are a very well-characterized example of store-operated Ca(2+) channels, so designated because they open when the endoplasmic reticulum (ER) Ca(2+) store becomes depleted. Physiologically, Ca(2+) is released from the ER lumen into the cytoplasm when activated receptors couple to phospholipase C and trigger production of the second messenger inositol 1,4,5-trisphosphate (IP(3)). IP(3) binds to IP(3) receptors in the ER membrane and activates Ca(2+) release. The proteins STIM and ORAI were discovered through limited and genome-wide RNAi screens, respectively, performed in Drosophila cells and focused on identifying modulators of store-operated Ca(2+) entry. STIM1 and STIM2 sense the depletion of ER Ca(2+) stores, whereas ORAI1 is a pore subunit of the CRAC channel. In this review, we discuss selected aspects of Ca(2+) signaling in cells of the immune system, focusing on the roles of STIM and ORAI proteins in store-operated Ca(2+) entry.
Figures




References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. - PubMed
-
- Di Capite J, Parekh AB. CRAC channels and Ca2+ signaling in mast cells. Immunol Rev. 2009;231:45–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

