A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis
- PMID: 20307214
- PMCID: PMC3158389
- DOI: 10.1089/jop.2009.0087
A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis
Abstract
Aims: To examine the efficacy of tacrolimus ophthalmic suspension 0.1% in treating severe allergic conjunctivitis.
Methods: This was a multicenter, randomized, double-masked, placebo-controlled clinical trial. Fifty-six patients with severe allergic conjunctivitis in whom topical antiallergic agents and corticosteroids had been ineffective were randomized to tacrolimus or placebo treatment. Patients were treated either with tacrolimus or placebo twice-daily for 4 weeks. Severity of objective signs in palpebral and bulbar conjunctiva, limbus, and corneal involvement was assessed using 4 grades. Seven subjective symptoms were evaluated by visual analog scale (VAS) assessment. The primary efficacy endpoint was change in the total score of objective signs at the end of treatment. The secondary efficacy endpoints included change in the score for each objective sign and change in the VAS for each subjective symptom. Safety was assessed based on the severity and the incidence of adverse events.
Results: Mean change from baseline in total score for objective signs was significantly greater in the tacrolimus (-5.6 + or - 5.1) than in the placebo group (-0.1 + or - 4.5; P < 0.001). Tacrolimus significantly improved giant papillae (P = 0.001) and corneal involvement (P = 0.005). Five subjective symptoms (itching, discharge, hyperemia, lacrimation, and foreign body sensation) were significantly better in the tacrolimus than in the placebo group. The most frequent treatment-related adverse event in the tacrolimus group was mild ocular irritation upon topical instillation, which was well-tolerated.
Conclusion: Tacrolimus ophthalmic suspension 0.1% is effective in treating severe allergic conjunctivitis.
Figures


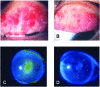

References
-
- Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107:1157–1163. - PubMed
-
- Tabbara K.F. Ocular complications of vernal keratoconjunctivitis. Can. J. Ophthalmol. 1999;34:88–92. - PubMed
-
- Foster C.S, Calonge M. Atopic keratoconjunctivitis. Ophthalmology. 1990;97:992–1000. - PubMed
-
- BenEzra D, Pe’er J, Brodsky M, et al. Cyclosporine eyedrops for the treatment of severe vernal keratoconjunctivitis. Am. J. Ophthalmol. 1986;101:278–282. - PubMed
-
- BenEzra D, Matamoros N, Cohen E. Treatment of severe vernal keratoconjunctivitis with cyclosporine A eyedrops. Transplant. Proc. 1988;20:644–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
