Interferon-gamma inhibits interleukin-1beta-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis
- PMID: 20307272
- PMCID: PMC2888198
- DOI: 10.1186/ar2960
Interferon-gamma inhibits interleukin-1beta-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis
Abstract
Introduction: The first few months after symptom onset represents a pathologically distinct phase in rheumatoid arthritis (RA). We used relevant experimental models to define the pathological role of interferon-gamma (IFN-gamma) during early inflammatory arthritis.
Methods: We studied IFN-gamma's capacity to modulate interleukin-1beta (IL-1beta) induced degenerative responses using RA fibroblast-like synoviocytes (FLS), a bovine articular cartilage explant (BACE)/RA-FLS co-culture model and an experimental inflammatory arthritis model (murine antigen-induced arthritis (AIA)).
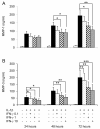
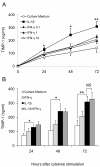
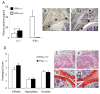
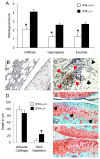
Results: IFN-gamma modulated IL-1beta driven matrix metalloproteinases (MMP) synthesis resulting in the down-regulation of MMP-1 and MMP-3 production in vitro. IFN-gamma did not affect IL-1beta induced tissue inhibitor of metalloproteinase-1 (TIMP-1) production by RA FLS but skewed the MMP/TIMP-1 balance sufficiently to attenuate glycosaminoglycan-depletion in our BACE model. IFN-gamma reduced IL-1beta expression in the arthritic joint and prevented cartilage degeneration on Day 3 of AIA.
Conclusions: Early therapeutic intervention with IFN-gamma may be critical to orchestrate tissue-protective responses during inflammatory arthritis.
Figures

References
-
- Ring GH, Dai Z, Saleem S, Baddoura FK, Lakkis FG. Increased susceptibility to immunologically mediated glomerulonephritis in IFN-gamma-deficient mice. J Immunol. 1999;163:2243–2248. - PubMed
-
- Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158:5997–6005. - PubMed
-
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. - PubMed
-
- Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167:5464–5469. - PubMed
-
- Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–5513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

