Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-kappaB signaling
- PMID: 20307321
- PMCID: PMC2859401
- DOI: 10.1186/1743-8977-7-6
Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-kappaB signaling
Abstract
Background: Epidemiological evidence supports the association between exposure to ambient particulate matter (PM) and cardiovascular diseases. Chronic exposure to ultrafine particles (UFP; Dp <100 nm) is reported to promote atherosclerosis in ApoE knockout mice. Atherogenesis-prone factors induce endothelial dysfunction that contributes to the initiation and progression of atherosclerosis. We previously demonstrated that UFP induced oxidative stress via c-Jun N-terminal Kinases (JNK) activation in endothelial cells. In this study, we investigated pro-inflammatory responses of human aortic endothelial cells (HAEC) exposed to UFP emitted from a diesel truck under an idling mode (UFP1) and an urban dynamometer driving schedule (UFP2), respectively. We hypothesize that UFP1 and UFP2 with distinct chemical compositions induce differential pro-inflammatory responses in endothelial cells.
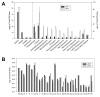
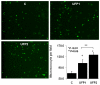
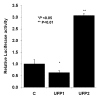
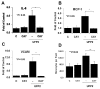
Results: UFP2 contained a higher level of redox active organic compounds and metals on a per PM mass basis than UFP1. While both UFP1 and UFP2 induced superoxide production and up-regulated stress response genes such as heme oxygenease-1 (HO-1), OKL38, and tissue factor (TF), only UFP2 induced the expression of pro-inflammatory genes such as IL-8 (2.8 +/- 0.3-fold), MCP-1 (3.9 +/- 0.4-fold), and VCAM (6.5 +/- 1.1-fold) (n = 3, P < 0.05). UFP2-exposed HAEC also bound to a higher number of monocytes than UFP1-exposed HAEC (Control = 70 +/- 7.5, UFP1 = 106.7 +/- 12.5, UFP2 = 137.0 +/- 8.0, n = 3, P < 0.05). Adenovirus NF-kappaB Luciferase reporter assays revealed that UFP2, but not UFP1, significantly induced NF-kappaB activities. NF-kappaB inhibitor, CAY10512, significantly abrogated UFP2-induced pro-inflammatory gene expression and monocyte binding.
Conclusion: While UFP1 induced higher level of oxidative stress and stress response gene expression, only UFP2, with higher levels of redox active organic compounds and metals, induced pro-inflammatory responses via NF-kappaB signaling. Thus, UFP with distinct chemical compositions caused differential response patterns in endothelial cells.
Figures



References
-
- Knutsen S, Shavlik D, Chen LH, Beeson WL, Ghamsary M, Petersen F. The association between ambient particulate air pollution levels and risk of cardiopulmonary and all-cause mortality during 22 years follow-up of a non-smoking cohort. Epidemiology. 2004;15:S45–S45. doi: 10.1097/00001648-200407000-00104. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

