A hazard analysis for a generic insulin infusion pump
- PMID: 20307387
- PMCID: PMC2864162
- DOI: 10.1177/193229681000400207
A hazard analysis for a generic insulin infusion pump
Abstract
Background: Researchers at the Food and Drug Administration (FDA)/Center for Device and Radiological Health/Office of Science and Engineering Laboratories have been exploring the concept of model-based engineering as a means for improving the quality of medical device software. Insulin pumps were chosen as a research subject because their design provides the desired degree of research complexity and these types of devices present an ongoing regulatory challenge.
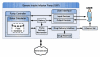
Methods: Insulin pump hazards and their contributing factors are considered in the context of a highly abstract generic insulin infusion pump (GIIP) model. Hazards were identified by consulting with manufacturers, pump users, and clinicians; by reviewing national and international standards and adverse event reports collected by the FDA; and from workshops sponsored by Diabetes Technology Society. This information has been consolidated in tabular form to facilitate further community analysis and discussion.
Results: A generic insulin infusion pump model architecture has been established. A fairly comprehensive hazard analysis document, corresponding to the GIIP model, is presented in this article.
Conclusions: We believe that this work represents the genesis of an insulin pump safety reference standard upon which future insulin pump designs can be based to help ensure a basic level of safety. More interaction with the diabetes community is needed to assure the quality of this safety modeling process.
(c) 2010 Diabetes Technology Society.
Figures
Similar articles
-
Generic safety requirements for developing safe insulin pump software.J Diabetes Sci Technol. 2011 Nov 1;5(6):1403-19. doi: 10.1177/193229681100500612. J Diabetes Sci Technol. 2011. PMID: 22226258 Free PMC article.
-
Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group.Diabetes Care. 2015 Apr;38(4):716-22. doi: 10.2337/dc15-0168. Diabetes Care. 2015. PMID: 25776138
-
Second insulin pump safety meeting: summary report.J Diabetes Sci Technol. 2010 Mar 1;4(2):488-93. doi: 10.1177/193229681000400232. J Diabetes Sci Technol. 2010. PMID: 20307411 Free PMC article.
-
Clinical review: insulin pump-associated adverse events in adults and children.Acta Diabetol. 2015 Dec;52(6):1017-24. doi: 10.1007/s00592-015-0784-2. Epub 2015 Jun 21. Acta Diabetol. 2015. PMID: 26092321 Review.
-
Pitfalls of insulin pump clocks: technical glitches that may potentially affect medical care in patients with diabetes.J Diabetes Sci Technol. 2014 Nov;8(6):1215-20. doi: 10.1177/1932296814541811. Epub 2014 Jul 2. J Diabetes Sci Technol. 2014. PMID: 25355713 Free PMC article. Review.
Cited by
-
Determination of Micromovements in Removable Prosthesis during Mastication: A Pilot Study with 3D Electromagnetic Articulography.Bioengineering (Basel). 2024 Feb 28;11(3):229. doi: 10.3390/bioengineering11030229. Bioengineering (Basel). 2024. PMID: 38534503 Free PMC article.
-
Detection of Insulin Pump Malfunctioning to Improve Safety in Artificial Pancreas Using Unsupervised Algorithms.J Diabetes Sci Technol. 2019 Nov;13(6):1065-1076. doi: 10.1177/1932296819881452. Epub 2019 Oct 14. J Diabetes Sci Technol. 2019. PMID: 31608660 Free PMC article.
-
NHLBI support of systems biology.Front Physiol. 2013 Nov 15;4:299. doi: 10.3389/fphys.2013.00299. eCollection 2013. Front Physiol. 2013. PMID: 24298257 Free PMC article.
-
Chewing Analysis by Means of Electromagnetic Articulography: Current Developments and New Possibilities.Sensors (Basel). 2023 Nov 30;23(23):9511. doi: 10.3390/s23239511. Sensors (Basel). 2023. PMID: 38067884 Free PMC article.
-
Performance of a Continuous Subcutaneous Insulin Infusion (CSII) Pump With Acoustic Volume and Flow Sensing in Simulated High-Consequence Situations.IEEE Open J Eng Med Biol. 2024 Jun 4;5:593-599. doi: 10.1109/OJEMB.2024.3408092. eCollection 2024. IEEE Open J Eng Med Biol. 2024. PMID: 39157058 Free PMC article.
References
-
- Diabetic Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. - PubMed
-
- ISO 14971. 2007. Medical devices–application of risk management to medical devices.
-
- FDA MAUDE database. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfMAUDE/search.CFM.
-
- Kampfner RR. Model-based development of computer-based information systems. Proceedings of the Workshop on Engineering of Computer-Based Systems (ECBS); 1997. p. 354.
-
- Arney D, Jetley R, Jones P, Lee I, Sokolsky O. Formal methods based development of a PCA infusion pump reference model: generic infusion pump (GIP) project. Joint Workshop on High Confidence Medical Devices, Software, and Systems and Medical Device Plug-and-Play Interoperability; 2007. pp. 22–33.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources