Blocking the K-pathway still allows rapid one-electron reduction of the binuclear center during the anaerobic reduction of the aa3-type cytochrome c oxidase from Rhodobacter sphaeroides
- PMID: 20307488
- PMCID: PMC2891110
- DOI: 10.1016/j.bbabio.2010.03.012
Blocking the K-pathway still allows rapid one-electron reduction of the binuclear center during the anaerobic reduction of the aa3-type cytochrome c oxidase from Rhodobacter sphaeroides
Abstract
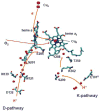
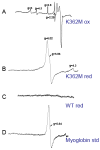
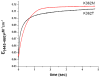
The K-pathway is one of the two proton-input channels required for function of cytochrome c oxidase. In the Rhodobacter sphaeroides cytochrome c oxidase, the K-channel starts at Glu101 in subunit II, which is at the surface of the protein exposed to the cytoplasm, and runs to Tyr288 at the heme a3/CuB active site. Mutations of conserved, polar residues within the K-channel block or inhibit steady state oxidase activity. A large body of research has demonstrated that the K-channel is required to fully reduce the heme/Cu binuclear center, prior to the reaction with O2, presumably by providing protons to stabilize the reduced metals (ferrous heme a3 and cuprous CuB). However, there are conflicting reports which raise questions about whether blocking the K-channel blocks both electrons or only one electron from reaching the heme/Cu center. In the current work, the rate and extent of the anaerobic reduction of the heme/Cu center were monitored by optical and EPR spectroscopies, comparing the wild type and mutants that block the K-channel. The new data show that when the K-channel is blocked, one electron will still readily enter the binuclear center. The one-electron reduction of the resting oxidized ("O") heme/Cu center of the K362M mutant, results in a partially reduced binuclear center in which the electron is distributed about evenly between heme a3 and CuB in the R. sphaeroides oxidase. Complete reduction of the heme/Cu center requires the uptake of two protons which must be delivered through the K-channel.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Pereira MM, Santana M, Teixeira M. A Novel Scenario for the Evoluation of Haem-copper Oxygen Reductases. Biochim Biophys Acta. 2001;1505:185–208. - PubMed
-
- Pereira MM, Sousa FL, Verissimo AF, Teixeira M. Looking for the minimum common denominator in haem-copper oxygen reductases: Towards a unified catalytic mechanism. Biochim Biophys Acta. 2008;1777:929–934. - PubMed
-
- Tsukihara T, Aoyama H, Yamashita E, Takashi T, Yamaguichi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The Whole Structure of the 13-Subunit Oxidized Cytochrome c Oxidase at 2.8 Å. Science. 1996;272:1136–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

