NPC1L1 and cholesterol transport
- PMID: 20307540
- PMCID: PMC2909875
- DOI: 10.1016/j.febslet.2010.03.030
NPC1L1 and cholesterol transport
Abstract
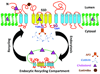
The polytopic transmembrane protein, Niemann-Pick C1-Like 1 (NPC1L1), is enriched in the apical membrane of small intestine absorptive enterocytes where it mediates extracellular sterol transport across the brush border membrane. It is essential for intestinal sterol absorption and is the molecular target of ezetimibe, a potent cholesterol absorption inhibitor that lowers blood cholesterol in humans. NPC1L1 is also highly expressed in human liver. The hepatic function of NPC1L1 may be to limit excessive biliary cholesterol loss. NPC1L1-dependent sterol uptake seems to be a clathrin-mediated endocytic process and is regulated by cellular cholesterol content. Recently, NPC1L1 inhibition has been shown to have beneficial effects on components of the metabolic syndrome, such as obesity, insulin resistance, and fatty liver, in addition to atherosclerosis.
Copyright 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport.Annu Rev Physiol. 2011;73:239-59. doi: 10.1146/annurev-physiol-012110-142233. Annu Rev Physiol. 2011. PMID: 20809793 Free PMC article. Review.
-
Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter.Biochim Biophys Acta. 2009 Jul;1791(7):679-83. doi: 10.1016/j.bbalip.2009.01.002. Epub 2009 Jan 19. Biochim Biophys Acta. 2009. PMID: 19272334 Review.
-
Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine.J Lipid Res. 2012 Oct;53(10):2092-2101. doi: 10.1194/jlr.M027359. Epub 2012 Jul 17. J Lipid Res. 2012. PMID: 22811412 Free PMC article.
-
Ezetimibe inhibits hepatic Niemann-Pick C1-Like 1 to facilitate macrophage reverse cholesterol transport in mice.Arterioscler Thromb Vasc Biol. 2013 May;33(5):920-5. doi: 10.1161/ATVBAHA.112.301187. Epub 2013 Mar 7. Arterioscler Thromb Vasc Biol. 2013. PMID: 23471229 Free PMC article.
-
[Progress of Niemann-Pick type C1 Like 1 on cholesterol metabolism].Sheng Li Xue Bao. 2012 Dec 25;64(6):721-8. Sheng Li Xue Bao. 2012. PMID: 23258338 Review. Chinese.
Cited by
-
Emerging applications of single-cell profiling in precision medicine of atherosclerosis.J Transl Med. 2024 Jan 23;22(1):97. doi: 10.1186/s12967-023-04629-y. J Transl Med. 2024. PMID: 38263066 Free PMC article. Review.
-
Association of rs2072183 SNP and serum lipid levels in the Mulao and Han populations.Lipids Health Dis. 2012 May 30;11:61. doi: 10.1186/1476-511X-11-61. Lipids Health Dis. 2012. PMID: 22646906 Free PMC article.
-
SREBP2 mediates the modulation of intestinal NPC1L1 expression by curcumin.Am J Physiol Gastrointest Liver Physiol. 2011 Jul;301(1):G148-55. doi: 10.1152/ajpgi.00119.2011. Epub 2011 Apr 28. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21527728 Free PMC article.
-
Ezetimibe Improves Rosuvastatin Effects on Inflammation and Vascular Endothelial Function in Acute Coronary Syndrome Patients Undergoing PCI.J Interv Cardiol. 2021 Sep 7;2021:2995602. doi: 10.1155/2021/2995602. eCollection 2021. J Interv Cardiol. 2021. PMID: 34566523 Free PMC article. Clinical Trial.
-
CUP-1 is a novel protein involved in dietary cholesterol uptake in Caenorhabditis elegans.PLoS One. 2012;7(3):e33962. doi: 10.1371/journal.pone.0033962. Epub 2012 Mar 27. PLoS One. 2012. PMID: 22479487 Free PMC article.
References
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. - PubMed
-
- Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis; Cold Spring Harb Symp Quant Biol; 2002. pp. 491–498. - PubMed
-
- Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

