A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus
- PMID: 20307593
- PMCID: PMC7115569
- DOI: 10.1016/j.vaccine.2010.03.008
A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus
Abstract
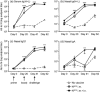
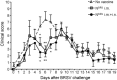
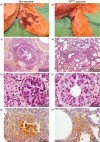
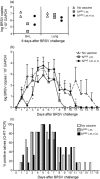
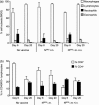
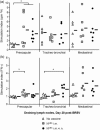
Human and bovine respiratory syncytial viruses (HRSV and BRSV) are two closely related, worldwide prevalent viruses that are the leading cause of severe airway disease in children and calves, respectively. Efficacy of commercial bovine vaccines needs improvement and no human vaccine is licensed yet. We reported that nasal vaccination with the HRSV nucleoprotein produced as recombinant ring-shaped nanoparticles (N(SRS)) protects mice against a viral challenge with HRSV. The aim of this work was to evaluate this new vaccine that uses a conserved viral antigen, in calves, natural hosts for BRSV. Calves, free of colostral or natural anti-BRSV antibodies, were vaccinated with N(SRS) either intramuscularly, or both intramuscularly and intranasally using Montanide ISA71 and IMS4132 as adjuvants and challenged with BRSV. All vaccinated calves developed anti-N antibodies in blood and nasal secretions and N-specific cellular immunity in local lymph nodes. Clinical monitoring post-challenge demonstrated moderate respiratory pathology with local lung tissue consolidations for the non-vaccinated calves that were significantly reduced in the vaccinated calves. Vaccinated calves had lower viral loads than the non-vaccinated control calves. Thus N(SRS) vaccination in calves provided cross-protective immunity against BRSV infection without adverse inflammatory reaction.
Figures

Similar articles
-
Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies.PLoS One. 2014 Jun 19;9(6):e100392. doi: 10.1371/journal.pone.0100392. eCollection 2014. PLoS One. 2014. PMID: 24945377 Free PMC article.
-
A Recombinant BCG Vaccine Is Safe and Immunogenic in Neonatal Calves and Reduces the Clinical Disease Caused by the Respiratory Syncytial Virus.Front Immunol. 2021 Apr 26;12:664212. doi: 10.3389/fimmu.2021.664212. eCollection 2021. Front Immunol. 2021. PMID: 33981309 Free PMC article.
-
Protection against bovine respiratory syncytial virus in calves vaccinated with adjuvanted modified live vaccine administered in the face of maternal antibody.Vaccine. 2020 Jan 10;38(2):298-308. doi: 10.1016/j.vaccine.2019.10.015. Epub 2019 Oct 24. Vaccine. 2020. PMID: 31668818
-
[Immunobiology of bovine respiratory syncytial virus infections].Tijdschr Diergeneeskd. 1998 Nov 15;123(22):658-62. Tijdschr Diergeneeskd. 1998. PMID: 9836385 Review. Dutch.
-
Bovine respiratory syncytial virus.Vet Clin North Am Food Anim Pract. 2010 Jul;26(2):323-33. doi: 10.1016/j.cvfa.2010.04.010. Vet Clin North Am Food Anim Pract. 2010. PMID: 20619187 Review.
Cited by
-
Immunogenicity and Protective Potential of Mucosal Vaccine Formulations Based on Conserved Epitopes of Influenza A Viruses Fused to an Innovative Ring Nanoplatform in Mice and Chickens.Front Immunol. 2021 Nov 11;12:772550. doi: 10.3389/fimmu.2021.772550. eCollection 2021. Front Immunol. 2021. PMID: 34868036 Free PMC article.
-
Purification, stability, and immunogenicity analyses of five bluetongue virus proteins for use in development of a subunit vaccine that allows differentiation of infected from vaccinated animals.Clin Vaccine Immunol. 2014 Mar;21(3):443-52. doi: 10.1128/CVI.00776-13. Epub 2014 Jan 22. Clin Vaccine Immunol. 2014. PMID: 24451327 Free PMC article.
-
Preparation of mucosal nanoparticles and polymer-based inactivated vaccine for Newcastle disease and H9N2 AI viruses.Vet World. 2017 Feb;10(2):187-193. doi: 10.14202/vetworld.2017.187-193. Epub 2017 Feb 14. Vet World. 2017. PMID: 28344402 Free PMC article.
-
Characterization of an experimental vaccine for bovine respiratory syncytial virus.Clin Vaccine Immunol. 2014 Jul;21(7):997-1004. doi: 10.1128/CVI.00162-14. Epub 2014 May 14. Clin Vaccine Immunol. 2014. PMID: 24828093 Free PMC article.
-
First demonstration of the circulation of a pneumovirus in French pigs by detection of anti-swine orthopneumovirus nucleoprotein antibodies.Vet Res. 2018 Dec 5;49(1):118. doi: 10.1186/s13567-018-0615-x. Vet Res. 2018. PMID: 30518406 Free PMC article.
References
-
- Meyer G., Deplanche M., Schelcher F. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis. 2008;31(2–3):191–225. - PubMed
-
- Valarcher J.F., Taylor G. Bovine respiratory syncytial virus infection. Vet Res. 2007;38(2):153–180. - PubMed
-
- Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Vet Rec. 1996;138(5):101–105. - PubMed
-
- Openshaw P.J., Yamaguchi Y., Tregoning J.S. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol. 2004;114(6):1275–1277. - PubMed
-
- Kapikian A.Z., Mitchell R.H., Chanock R.M., Shvedoff R.A., Stewart C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89(4):405–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

