Comparison of egg and high yielding MDCK cell-derived live attenuated influenza virus for commercial production of trivalent influenza vaccine: in vitro cell susceptibility and influenza virus replication kinetics in permissive and semi-permissive cells
- PMID: 20307595
- PMCID: PMC7172923
- DOI: 10.1016/j.vaccine.2010.03.005
Comparison of egg and high yielding MDCK cell-derived live attenuated influenza virus for commercial production of trivalent influenza vaccine: in vitro cell susceptibility and influenza virus replication kinetics in permissive and semi-permissive cells
Abstract
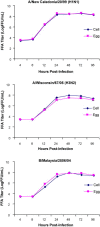
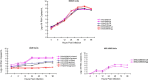
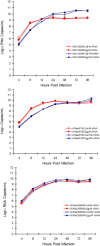
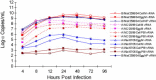
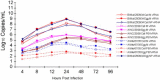
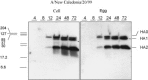
Currently MedImmune manufactures cold-adapted (ca) live, attenuated influenza vaccine (LAIV) from specific-pathogen free (SPF) chicken eggs. Difficulties in production scale-up and potential exposure of chicken flocks to avian influenza viruses especially in the event of a pandemic influenza outbreak have prompted evaluation and development of alternative non-egg based influenza vaccine manufacturing technologies. As part of MedImmune's effort to develop the live attenuated influenza vaccine (LAIV) using cell culture production technologies we have investigated the use of high yielding, cloned MDCK cells as a substrate for vaccine production by assessing host range and virus replication of influenza virus produced from both SPF egg and MDCK cell production technologies. In addition to cloned MDCK cells the indicator cell lines used to evaluate the impact of producing LAIV in cells on host range and replication included two human cell lines: human lung carcinoma (A549) cells and human muco-epidermoid bronchiolar carcinoma (NCI H292) cells. The influenza viruses used to infect the indicators cell lines represented both the egg and cell culture manufacturing processes and included virus strains that composed the 2006-2007 influenza seasonal trivalent vaccine (A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/05 (H3N2) and B/Malaysia/2506/04). Results from this study demonstrate remarkable similarity between influenza viruses representing the current commercial egg produced and developmental MDCK cell produced vaccine production platforms. MedImmune's high yielding cloned MDCK cells used for the cell culture based vaccine production were highly permissive to both egg and cell produced ca attenuated influenza viruses. Both the A549 and NCI H292 cells regardless of production system were less permissive to influenza A and B viruses than the MDCK cells. Irrespective of the indicator cell line used the replication properties were similar between egg and the cell produced influenza viruses. Based on these study results we conclude that the MDCK cell produced and egg produced vaccine strains are highly comparable.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Belshe R.B., Nichol K.L., Black S.B., Shinefield H., Cordova J., Walker R. Safety, efficacy and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004;39:920–927. - PubMed
-
- Belshe R.B., Lee M.-S., Walker R.E., Stoddard J., Mendelman P.M. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vac. 2004;3(6):643–654. - PubMed
-
- Nichol K.L. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–1775. - PubMed
-
- Mathews J. Egg-based influenza vaccine production—30 years of commercial experience and our future expectations for cell culture. National academy of engineering and institute of medicine conference, vaccine production engineering approaches to a pandemic; Washington, DC, April 10–11; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

