A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor
- PMID: 20308075
- PMCID: PMC2871448
- DOI: 10.1074/jbc.M110.101980
A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor
Abstract
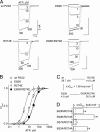
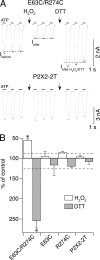
The recent crystal structure of the ATP-gated P2X4 receptor revealed a static view of its architecture, but the molecular mechanisms underlying the P2X channels activation are still unknown. By using a P2X2 model based on the x-ray structure, we sought salt bridges formed between charged residues located in a region that directly connects putative ATP-binding sites to the ion channel. To reveal their significance for ion channel activation, we made systematic charge exchanges and measured the effects on ATP sensitivity. We found that charge reversals at the interfacial residues Glu(63) and Arg(274) produced gain-of-function phenotypes that were cancelled upon paired charge swapping. These results suggest that a putative intersubunit salt bridge formed between Glu(63) and Arg(274) contributes to the ion channel function. Engineered cysteines E63C and R274C formed redox-dependent cross-links in the absence of ATP. By contrast, the presence of ATP reduced the rate of disulfide bond formation, indicating that ATP binding might trigger relative movement of adjacent subunits at the level of Glu(63) and Arg(274), allowing the transmembrane helices to open the channel.
Figures


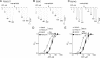


Similar articles
-
Subunit arrangement in P2X receptors.J Neurosci. 2003 Oct 1;23(26):8903-10. doi: 10.1523/JNEUROSCI.23-26-08903.2003. J Neurosci. 2003. PMID: 14523092 Free PMC article.
-
On the role of the first transmembrane domain in cation permeability and flux of the ATP-gated P2X2 receptor.J Biol Chem. 2008 Feb 22;283(8):5110-7. doi: 10.1074/jbc.M708713200. Epub 2007 Nov 29. J Biol Chem. 2008. PMID: 18048351
-
Functional and structural identification of amino acid residues of the P2X2 receptor channel critical for the voltage- and [ATP]-dependent gating.J Physiol. 2009 Dec 15;587(Pt 24):5801-18. doi: 10.1113/jphysiol.2009.182824. J Physiol. 2009. PMID: 19884318 Free PMC article.
-
Molecular properties of P2X receptors.Pflugers Arch. 2006 Aug;452(5):486-500. doi: 10.1007/s00424-006-0073-6. Epub 2006 Apr 11. Pflugers Arch. 2006. PMID: 16607539 Review.
-
Biophysics of P2X receptors.Pflugers Arch. 2006 Aug;452(5):501-12. doi: 10.1007/s00424-006-0078-1. Epub 2006 May 13. Pflugers Arch. 2006. PMID: 16708237 Review.
Cited by
-
Highly conserved salt bridge stabilizes rigid signal patch at extracellular loop critical for surface expression of acid-sensing ion channels.J Biol Chem. 2012 Apr 27;287(18):14443-55. doi: 10.1074/jbc.M111.334250. Epub 2012 Mar 7. J Biol Chem. 2012. PMID: 22399291 Free PMC article.
-
Molecular determinants for agonist recognition and discrimination in P2X2 receptors.J Gen Physiol. 2019 Jul 1;151(7):898-911. doi: 10.1085/jgp.201912347. Epub 2019 May 24. J Gen Physiol. 2019. PMID: 31126967 Free PMC article.
-
A Highly Conserved Salt Bridge Stabilizes the Kinked Conformation of β2,3-Sheet Essential for Channel Function of P2X4 Receptors.J Biol Chem. 2016 Apr 8;291(15):7990-8003. doi: 10.1074/jbc.M115.711127. Epub 2016 Feb 10. J Biol Chem. 2016. PMID: 26865631 Free PMC article.
-
Size matters in activation/inhibition of ligand-gated ion channels.Trends Pharmacol Sci. 2012 Sep;33(9):482-93. doi: 10.1016/j.tips.2012.06.005. Epub 2012 Jul 11. Trends Pharmacol Sci. 2012. PMID: 22789930 Free PMC article. Review.
-
Structural and Functional Analysis of Gly212 Mutants Reveals the Importance of Intersubunit Interactions in ASIC1a Channel Function.Front Mol Biosci. 2020 Apr 28;7:58. doi: 10.3389/fmolb.2020.00058. eCollection 2020. Front Mol Biosci. 2020. PMID: 32411719 Free PMC article.
References
-
- Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 - PubMed
-
- Evans R. J., Derkach V., Surprenant A. (1992) Nature 357, 503–505 - PubMed
-
- Bo X., Alavi A., Xiang Z., Oglesby I., Ford A., Burnstock G. (1999) Neuroreport 10, 1107–1111 - PubMed
-
- Finger T. E., Danilova V., Barrows J., Bartel D. L., Vigers A. J., Stone L., Hellekant G., Kinnamon S. C. (2005) Science 310, 1495–1499 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

