Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging
- PMID: 20308077
- PMCID: PMC2871433
- DOI: 10.1074/jbc.M109.077503
Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging
Abstract
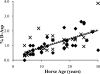
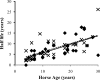
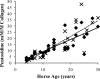
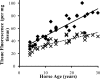
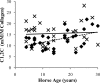
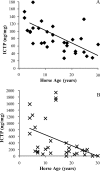
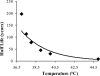
Little is known about the rate at which protein turnover occurs in living tendon and whether the rate differs between tendons with different physiological roles. In this study, we have quantified the racemization of aspartic acid to calculate the age of the collagenous and non-collagenous components of the high strain injury-prone superficial digital flexor tendon (SDFT) and low strain rarely injured common digital extensor tendon (CDET) in a group of horses with a wide age range. In addition, the turnover of collagen was assessed indirectly by measuring the levels of collagen degradation markers (collagenase-generated neoepitope and cross-linked telopeptide of type I collagen). The fractional increase in D-Asp was similar (p = 0.7) in the SDFT (5.87 x 10(-4)/year) and CDET (5.82 x 10(-4)/year) tissue, and D/L-Asp ratios showed a good correlation with pentosidine levels. We calculated a mean (+/-S.E.) collagen half-life of 197.53 (+/-18.23) years for the SDFT, which increased significantly with horse age (p = 0.03) and was significantly (p < 0.001) higher than that for the CDET (34.03 (+/-3.39) years). Using similar calculations, the half-life of non-collagenous protein was 2.18 (+/-0.41) years in the SDFT and was significantly (p = 0.04) lower than the value of 3.51 (+/-0.51) years for the CDET. Collagen degradation markers were higher in the CDET and suggested an accumulation of partially degraded collagen within the matrix with aging in the SDFT. We propose that increased susceptibility to injury in older individuals results from an inability to remove partially degraded collagen from the matrix leading to reduced mechanical competence.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

