Atomic layer deposition-based functionalization of materials for medical and environmental health applications
- PMID: 20308114
- PMCID: PMC2944392
- DOI: 10.1098/rsta.2010.0011
Atomic layer deposition-based functionalization of materials for medical and environmental health applications
Abstract
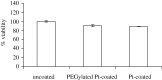
Nanoporous alumina membranes exhibit high pore densities, well-controlled and uniform pore sizes, as well as straight pores. Owing to these unusual properties, nanoporous alumina membranes are currently being considered for use in implantable sensor membranes and water purification membranes. Atomic layer deposition is a thin-film growth process that may be used to modify the pore size in a nanoporous alumina membrane while retaining a narrow pore distribution. In addition, films deposited by means of atomic layer deposition may impart improved biological functionality to nanoporous alumina membranes. In this study, zinc oxide coatings and platinum coatings were deposited on nanoporous alumina membranes by means of atomic layer deposition. PEGylated nanoporous alumina membranes were prepared by self-assembly of 1-mercaptoundec-11-yl hexa(ethylene glycol) on platinum-coated nanoporous alumina membranes. The pores of the PEGylated nanoporous alumina membranes remained free of fouling after exposure to human platelet-rich plasma; protein adsorption, fibrin networks and platelet aggregation were not observed on the coated membrane surface. Zinc oxide-coated nanoporous alumina membranes demonstrated activity against two waterborne pathogens, Escherichia coli and Staphylococcus aureus. The results of this work indicate that nanoporous alumina membranes may be modified using atomic layer deposition for use in a variety of medical and environmental health applications.
Figures




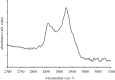

 Springer Science+Business Media.)
Springer Science+Business Media.)


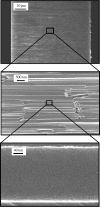




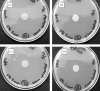
Similar articles
-
Cytotoxicity of cultured macrophages exposed to antimicrobial zinc oxide (ZnO) coatings on nanoporous aluminum oxide membranes.Biomatter. 2013 Jul-Sep;3(3):e25528. doi: 10.4161/biom.25528. Epub 2013 Jun 25. Biomatter. 2013. PMID: 23881040 Free PMC article.
-
Nanoporous Aluminum Oxide Membranes Coated with Atomic Layer Deposition-Grown Titanium Dioxide for Biomedical Applications: An In Vitro Evaluation.J Biomed Nanotechnol. 2015 Dec;11(12):2275-85. doi: 10.1166/jbn.2015.2169. J Biomed Nanotechnol. 2015. PMID: 26510320
-
Surface modification of nanoporous alumina surfaces with poly(ethylene glycol).Langmuir. 2004 Sep 14;20(19):8035-41. doi: 10.1021/la049075x. Langmuir. 2004. PMID: 15350069
-
Advances in Optical Biosensors and Sensors Using Nanoporous Anodic Alumina.Sensors (Basel). 2020 Sep 7;20(18):5068. doi: 10.3390/s20185068. Sensors (Basel). 2020. PMID: 32906635 Free PMC article. Review.
-
Recent Advances in Nanoporous Membranes for Water Purification.Nanomaterials (Basel). 2018 Jan 25;8(2):65. doi: 10.3390/nano8020065. Nanomaterials (Basel). 2018. PMID: 29370128 Free PMC article. Review.
Cited by
-
New development of atomic layer deposition: processes, methods and applications.Sci Technol Adv Mater. 2019 May 23;20(1):465-496. doi: 10.1080/14686996.2019.1599694. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 31164953 Free PMC article. Review.
-
Nanoporous solid-state membranes modified with multi-wall carbon nanotubes with anti-biofouling property.Int J Nanomedicine. 2019 Mar 5;14:1669-1685. doi: 10.2147/IJN.S189728. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30880972 Free PMC article.
-
Genotoxicity of Aluminum and Aluminum Oxide Nanomaterials in Rats Following Oral Exposure.Nanomaterials (Basel). 2020 Feb 11;10(2):305. doi: 10.3390/nano10020305. Nanomaterials (Basel). 2020. PMID: 32053952 Free PMC article.
-
Atomic layer deposition on flexible polymeric materials for lithium-ion batteries.RSC Adv. 2025 Apr 17;15(16):12382-12401. doi: 10.1039/d5ra00652j. eCollection 2025 Apr 16. RSC Adv. 2025. PMID: 40248234 Free PMC article. Review.
-
Sub-nanometer atomic layer deposition for spintronics in magnetic tunnel junctions based on graphene spin-filtering membranes.ACS Nano. 2014 Aug 26;8(8):7890-5. doi: 10.1021/nn5017549. ACS Nano. 2014. PMID: 24988469 Free PMC article.
References
-
- Adiga S. P., et al. Nanoporous materials for biomedical devices. J. Miner. Metals Mater. Soc. 2008;61:26–32.
-
- Akhavan O., Mehrabian M., Mirabbaszadeh K., Azimirad R. Hydrothermal synthesis of ZnO nanorod arrays for photocatalytic inactivation of bacteria. J. Phys. D. 2009;42 doi: 10.1088//42/22/225305. 225 305 ( ) - DOI
-
- Andara M., et al. Hemocompatibility of diamondlike carbon-metal composite thin films. Diamond Related Mater. 2006;15:1941–1948. ( doi:1016/j.diamond.2006.05.013)
-
- Anderson S. H. C., Elliott H., Wallis D. J., Canham L. T., Powell J. J. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. Phys. Status Solidi A. 2003;197:331–335. ( doi:1002/pssa.06519)
-
- Anglin E. J., Cheng L. Y., Freeman W. R., Sailor M. J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008;60:1266–1277. ( doi:1016/j.addr.2008.03.017) - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

