An in vivo tethered toxin approach for the cell-autonomous inactivation of voltage-gated sodium channel currents in nociceptors
- PMID: 20308253
- PMCID: PMC2887988
- DOI: 10.1113/jphysiol.2010.187112
An in vivo tethered toxin approach for the cell-autonomous inactivation of voltage-gated sodium channel currents in nociceptors
Abstract
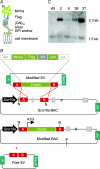
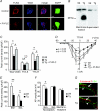
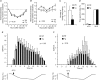
Understanding information flow in sensory pathways requires cell-selective approaches to manipulate the activity of defined neurones. Primary afferent nociceptors, which detect painful stimuli, are enriched in specific voltage-gated sodium channel (VGSC) subtypes. Toxins derived from venomous animals can be used to dissect the contributions of particular ion currents to cell physiology. Here we have used a transgenic approach to target a membrane-tethered isoform of the conotoxin MrVIa (t-MrVIa) only to nociceptive neurones in mice. T-MrVIa transgenic mice show a 44 +/- 7% reduction of tetrodotoxin-resistant (TTX-R) VGSC current densities. This inhibition is permanent, reversible and does not result in functional upregulation of TTX-sensitive (TTX-S) VGSCs, voltage-gated calcium channels (VGCCs) or transient receptor potential (TRP) channels present in nociceptive neurones. As a consequence of the reduction of TTX-R VGSC currents, t-MrVIa transgenic mice display decreased inflammatory mechanical hypersensitivity, cold pain insensitivity and reduced firing of cutaneous C-fibres sensitive to noxious cold temperatures. These data validate the use of genetically encoded t-toxins as a powerful tool to manipulate VGSCs in specific cell types within the mammalian nervous system. This novel genetic methodology can be used for circuit mapping and has the key advantage that it enables the dissection of the contribution of specific ionic currents to neuronal function and to behaviour.
Figures

Comment in
-
Tethered-toxin debut gets cold reception.J Physiol. 2010 May 15;588(Pt 10):1663. doi: 10.1113/jphysiol.2010.190736. J Physiol. 2010. PMID: 20472900 Free PMC article. No abstract available.
Similar articles
-
Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs.J Physiol. 2008 Mar 1;586(5):1321-36. doi: 10.1113/jphysiol.2007.146365. Epub 2008 Jan 10. J Physiol. 2008. PMID: 18187475 Free PMC article.
-
Inhibition of voltage-gated Na(+) currents in sensory neurones by the sea anemone toxin APETx2.Br J Pharmacol. 2012 Apr;165(7):2167-77. doi: 10.1111/j.1476-5381.2011.01674.x. Br J Pharmacol. 2012. PMID: 21943094 Free PMC article.
-
Celecoxib inhibits Na+ currents in rat dorsal root ganglion neurons.Brain Res. 2007 May 7;1148:53-61. doi: 10.1016/j.brainres.2007.02.023. Epub 2007 Feb 21. Brain Res. 2007. PMID: 17359944
-
The pharmacology of voltage-gated sodium channels in sensory neurones.Handb Exp Pharmacol. 2009;(194):519-61. doi: 10.1007/978-3-540-79090-7_15. Handb Exp Pharmacol. 2009. PMID: 19655117 Review.
-
Conotoxin modulation of voltage-gated sodium channels.Int J Biochem Cell Biol. 2008;40(11):2363-8. doi: 10.1016/j.biocel.2007.08.017. Epub 2007 Sep 14. Int J Biochem Cell Biol. 2008. PMID: 17951097 Review.
Cited by
-
Historical Perspective of the Characterization of Conotoxins Targeting Voltage-Gated Sodium Channels.Mar Drugs. 2023 Mar 27;21(4):209. doi: 10.3390/md21040209. Mar Drugs. 2023. PMID: 37103349 Free PMC article. Review.
-
Tethering toxins and peptide ligands for modulation of neuronal function.Curr Opin Neurobiol. 2012 Feb;22(1):72-8. doi: 10.1016/j.conb.2011.11.003. Epub 2011 Nov 24. Curr Opin Neurobiol. 2012. PMID: 22119144 Free PMC article. Review.
-
Effect of Percutaneous Electric Stimulation with High-Frequency Alternating Currents on the Sensory-Motor System of Healthy Volunteers: A Double-Blind Randomized Controlled Study.J Clin Med. 2022 Mar 25;11(7):1832. doi: 10.3390/jcm11071832. J Clin Med. 2022. PMID: 35407438 Free PMC article.
-
Remote control of neuronal signaling.Pharmacol Rev. 2011 Jun;63(2):291-315. doi: 10.1124/pr.110.003020. Epub 2011 Mar 17. Pharmacol Rev. 2011. PMID: 21415127 Free PMC article. Review.
-
The biochemical anatomy of cortical inhibitory synapses.PLoS One. 2012;7(6):e39572. doi: 10.1371/journal.pone.0039572. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768092 Free PMC article.
References
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
-
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. - PMC - PubMed
-
- Auer S, Stürzebecher AS, Jüttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibañez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

