Interactions of Encephalitozoon cuniculi polar tube proteins
- PMID: 20308291
- PMCID: PMC2876541
- DOI: 10.1128/IAI.01205-09
Interactions of Encephalitozoon cuniculi polar tube proteins
Abstract
Microsporidia are eukaryotic, obligate intracellular organisms defined by small spores that contain a single invasion organelle, the polar tube, which coils around the interior of the spore. When these parasites infect host cells, the polar tube is discharged from the anterior pole of the spore, pierces the cell, and transfers sporoplasm into the cytoplasm of the host. Three polar tube proteins (PTP1, PTP2, and PTP3) have been identified in this structure. The interactions of these proteins in the assembly and function of the polar tube are not known. This study was undertaken to examine the protein interactions of the Encephalitozoon cuniculi polar tube proteins (EcPTPs). Immunofluorescence and immunoelectron microscopy confirmed the colocalization of EcPTP1, EcPTP2, and EcPTP3 to the polar tube. Experiments using cross-linkers indicated that EcPTP1, EcPTP2, and EcPTP3 form a complex in the polar tube, which was confirmed by immunoprecipitation using EcPTP1 antiserum. Yeast two-hybrid analysis revealed that full-length EcPTP1, EcPTP2, and EcPTP3 interact with each other in vivo. Both the N and C termini of EcPTP1 were involved in these interactions, but the central region of this protein, which contains a repetitive motif, was not. Further studies of polar tube proteins and their structural interactions may help elucidate the formation of the polar tube during the invasion process.
Figures
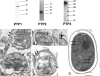
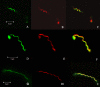




Similar articles
-
The microsporidian polar tube: a highly specialised invasion organelle.Int J Parasitol. 2005 Aug;35(9):941-53. doi: 10.1016/j.ijpara.2005.04.003. Int J Parasitol. 2005. PMID: 16005007 Free PMC article. Review.
-
The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi.Mol Biochem Parasitol. 2002 Jun;122(1):69-80. doi: 10.1016/s0166-6851(02)00073-7. Mol Biochem Parasitol. 2002. PMID: 12076771
-
Glycosylation of the major polar tube protein of Encephalitozoon cuniculi.Parasitol Res. 2010 Aug;107(3):761-4. doi: 10.1007/s00436-010-1950-7. Epub 2010 Jun 17. Parasitol Res. 2010. PMID: 20556427 Free PMC article.
-
Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species.Infect Immun. 2001 Feb;69(2):1016-24. doi: 10.1128/IAI.69.2.1016-1024.2001. Infect Immun. 2001. PMID: 11159998 Free PMC article.
-
Microsporidia: emerging pathogenic protists.Acta Trop. 2001 Feb 23;78(2):89-102. doi: 10.1016/s0001-706x(00)00178-9. Acta Trop. 2001. PMID: 11230819 Review.
Cited by
-
Identification and characterization a novel polar tube protein (NbPTP6) from the microsporidian Nosema bombycis.Parasit Vectors. 2020 Sep 15;13(1):475. doi: 10.1186/s13071-020-04348-z. Parasit Vectors. 2020. PMID: 32933572 Free PMC article.
-
Interaction between SWP9 and Polar Tube Proteins of the Microsporidian Nosema bombycis and Function of SWP9 as a Scaffolding Protein Contribute to Polar Tube Tethering to the Spore Wall.Infect Immun. 2017 Feb 23;85(3):e00872-16. doi: 10.1128/IAI.00872-16. Print 2017 Mar. Infect Immun. 2017. PMID: 28031263 Free PMC article.
-
The microsporidian polar tube: origin, structure, composition, function, and application.Parasit Vectors. 2023 Aug 30;16(1):305. doi: 10.1186/s13071-023-05908-9. Parasit Vectors. 2023. PMID: 37649053 Free PMC article. Review.
-
Ultrastructural insights into the microsporidian infection apparatus reveal the kinetics and morphological transitions of polar tube and cargo during host cell invasion.PLoS Biol. 2024 Feb 29;22(2):e3002533. doi: 10.1371/journal.pbio.3002533. eCollection 2024 Feb. PLoS Biol. 2024. PMID: 38422169 Free PMC article.
-
The role of microsporidian polar tube protein 4 (PTP4) in host cell infection.PLoS Pathog. 2017 Apr 20;13(4):e1006341. doi: 10.1371/journal.ppat.1006341. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28426751 Free PMC article.
References
-
- Cali, A., L. M. Weiss, and P. M. Takvorian. 2002. Brachiola algerae spore membrane systems, their activity during extrusion, and a new structural entity, the multilayered interlaced network, associated with the polar tube and the sporoplasm. J. Eukaryot. Microbiol. 49:164-174. - PubMed
-
- Delbac, F., F. Duffieux, D. David, G. Metenier, and C. P. Vivares. 1998. Immunocytochemical identification of spore proteins in two microsporidia, with emphasis on extrusion apparatus. J. Eukaryot. Microbiol. 45:224-231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

