Lack of interleukin-12 in p40-deficient mice leads to poor CD8+ T-cell immunity against Encephalitozoon cuniculi infection
- PMID: 20308292
- PMCID: PMC2876566
- DOI: 10.1128/IAI.00753-09
Lack of interleukin-12 in p40-deficient mice leads to poor CD8+ T-cell immunity against Encephalitozoon cuniculi infection
Abstract
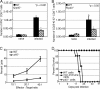
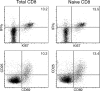
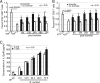
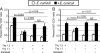
A CD8(+) T-cell response is critical for protection against Encephalitozoon cuniculi infection. However, the factors responsible for the generation of CD8(+) T-cell immunity during E. cuniculi infection and the cytokines involved in this process have not been identified. In the present study, we demonstrated that p40-deficient animals, which are unable to produce interleukin-12 (IL-12), have a serious defect in expansion of the CD8(+) T-cell response which compromises the survival of an infected host. Adoptive transfer of CD8(+) T cells from immunocompetent donors protected SCID mice infected with E. cuniculi, whereas administration of CD8(+) T cells from p40(-/-) mice failed to protect infected SCID mice. In vitro dendritic cell (DC) cultures from knockout mice pulsed with E. cuniculi spores were unable to develop a robust CD8(+) T-cell immune response. Addition of exogenous IL-12 or transfer of CD8(+) T cells that were initially primed with DC from p40(-/-) animals to DC cultures from immunocompetent mice (directly or via transwells) led to optimal expansion of these cells. This IL-12-mediated reinstatement of CD8(+) T-effector immunity was independent of gamma interferon (IFN-gamma) as addition of antibody to the cultures failed to have an effect. These studies demonstrated that IL-12 plays a predominant role in the expansion of effector CD8(+) T-cell immunity against E. cuniculi, which is critical for host survival. These findings are very important for understanding the protective immune mechanisms needed to protect an immunocompromised host against an opportunistic infection and can be extended to other microsporidial pathogens.
Figures




References
-
- Braunfuchsova, P., J. Salat, and J. Kopecky. 2001. CD8+ T lymphocytes protect SCID mice against Encephalitozoon cuniculi infection. Int. J. Parasitol. 31:681-686. - PubMed
-
- Braunfuchsova, P., J. Salat, and J. Kopecky. 2002. Comparison of the significance of CD4+ and CD8+ T lymphocytes in the protection of mice against Encephalitozoon cuniculi infection. J. Parasitol. 88:797-799. - PubMed
-
- Cocco, C., V. Pistoia, and I. Airoldi. 2009. New perspectives for melanoma immunotherapy: role of IL-12. Curr. Mol. Med. 9:459-469. - PubMed
-
- Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

