Trimer stability of YadA is critical for virulence of Yersinia enterocolitica
- PMID: 20308293
- PMCID: PMC2876551
- DOI: 10.1128/IAI.01350-09
Trimer stability of YadA is critical for virulence of Yersinia enterocolitica
Abstract
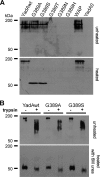
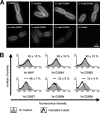
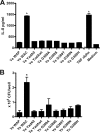
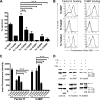
Yersinia adhesin A (YadA) is a trimeric autotransporter adhesin with multiple functions in host-pathogen interactions. The aim of this study was to dissect the virulence functions promoted by YadA in vitro and in vivo. To accomplish this, we generated Yersinia enterocolitica O:8 mutants expressing point mutations in YadA G389, a highly conserved residue in the membrane anchor of YadA, and analyzed their impact on YadA expression and virulence functions. We found that point mutations of YadA G389 led to impaired transport, stability, and surface display of YadA. YadA G389A and G389S mutants showed comparable YadA surface expression, autoagglutination, and adhesion to those of wild-type YadA but displayed reduced trimer stability and complement resistance in vitro and were 10- to 1,000-fold attenuated in experimental Y. enterocolitica infection in mice. The G389T, G389N, and G389H mutants lost trimer stability, exhibited strongly reduced surface display, autoagglutination, adhesion properties, and complement resistance, and were avirulent (>10,000-fold attenuation) in mice. Our data demonstrate that G389 is a critical residue of YadA, required for optimal trimer stability, transport, surface display, and serum resistance. We also show that stable trimeric YadA protein is essential for virulence of Y. enterocolitica.
Figures



References
-
- Ackermann, N., M. Tiller, G. Anding, A. Roggenkamp, and J. Heesemann. 2008. Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J. Bacteriol. 190:5031-5043. - PMC - PubMed
-
- Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. - PubMed
-
- Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52:451-469. - PubMed
-
- Bernstein, H. D. 2007. Are bacterial ‘autotransporters’ really transporters? Trends Microbiol. 15:441-447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

