Modulation of allergic airway inflammation by the oral pathogen Porphyromonas gingivalis
- PMID: 20308298
- PMCID: PMC2876545
- DOI: 10.1128/IAI.01270-09
Modulation of allergic airway inflammation by the oral pathogen Porphyromonas gingivalis
Abstract
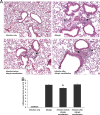
Accumulating evidence suggests that bacteria associated with periodontal disease may exert systemic immunomodulatory effects. Although the improvement in oral hygiene practices in recent decades correlates with the increased incidence of asthma in developed nations, it is not known whether diseases of the respiratory system might be influenced by the presence of oral pathogens. The present study sought to determine whether subcutaneous infection with the anaerobic oral pathogen Porphyromonas gingivalis exerts a regulatory effect on allergic airway inflammation. BALB/c mice sensitized and subsequently challenged with ovalbumin exhibited airway hyperresponsiveness to methacholine aerosol and increased airway inflammatory cell influx and Th2 cytokine (interleukin-4 [IL-4], IL-5, and IL-13) content relative to those in nonallergic controls. Airway inflammatory cell and cytokine contents were significantly reduced by establishment of a subcutaneous infection with P. gingivalis prior to allergen sensitization, whereas serum levels of ovalbumin-specific IgE and airway responsiveness were not altered. Conversely, subcutaneous infection initiated after allergen sensitization did not alter inflammatory end points but did reduce airway responsiveness in spite of increased serum IgE levels. These data provide the first direct evidence of a regulatory effect of an oral pathogen on allergic airway inflammation and responsiveness. Furthermore, a temporal importance of the establishment of infection relative to allergen sensitization is demonstrated for allergic outcomes.
Figures



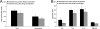
Similar articles
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Complete inhibition of allergic airway inflammation and remodelling in quadruple IL-4/5/9/13-/- mice.Clin Exp Allergy. 2007 Oct;37(10):1427-35. doi: 10.1111/j.1365-2222.2007.02789.x. Clin Exp Allergy. 2007. PMID: 17883722
-
Vbeta8+ T lymphocytes are essential in the regulation of airway hyperresponsiveness and bronchoalveolar eosinophilia but not in allergen-specific IgE in a murine model of allergic asthma.Clin Exp Allergy. 1998 Dec;28(12):1571-80. doi: 10.1046/j.1365-2222.1998.00387.x. Clin Exp Allergy. 1998. PMID: 10024230
-
Influenza A infection enhances antigen-induced airway inflammation and hyperresponsiveness in young but not aged mice.Clin Exp Allergy. 2014 Sep;44(9):1188-99. doi: 10.1111/cea.12365. Clin Exp Allergy. 2014. PMID: 25039815 Free PMC article.
-
Crude extracts of Caenorhabditis elegans suppress airway inflammation in a murine model of allergic asthma.PLoS One. 2012;7(4):e35447. doi: 10.1371/journal.pone.0035447. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558152 Free PMC article.
Cited by
-
Gram-negative microbiota is related to acute exacerbation in children with asthma.Clin Transl Allergy. 2021 Oct 12;11(8):e12069. doi: 10.1002/clt2.12069. eCollection 2021 Oct. Clin Transl Allergy. 2021. PMID: 34667591 Free PMC article.
-
Advances in the relationship between periodontopathogens and respiratory diseases (Review).Mol Med Rep. 2024 Mar;29(3):42. doi: 10.3892/mmr.2024.13166. Epub 2024 Jan 19. Mol Med Rep. 2024. PMID: 38240101 Free PMC article. Review.
-
Bacterial community profiles within the water samples of leptospirosis outbreak areas.PeerJ. 2024 Apr 29;12:e17096. doi: 10.7717/peerj.17096. eCollection 2024. PeerJ. 2024. PMID: 38699181 Free PMC article.
-
Relationships Between Oral Microecosystem and Respiratory Diseases.Front Mol Biosci. 2022 Jan 4;8:718222. doi: 10.3389/fmolb.2021.718222. eCollection 2021. Front Mol Biosci. 2022. PMID: 35071321 Free PMC article. Review.
-
Chlamydia pneumoniae infection induced allergic airway sensitization is controlled by regulatory T-cells and plasmacytoid dendritic cells.PLoS One. 2011;6(6):e20784. doi: 10.1371/journal.pone.0020784. Epub 2011 Jun 10. PLoS One. 2011. PMID: 21695198 Free PMC article.
References
-
- Beck, J. D., P. Eke, G. Heiss, P. Madianos, D. Couper, D. Lin, K. Moss, J. Elter, and S. Offenbacher. 2005. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation 112:19-24. - PubMed
-
- Brewer, J. P., A. B. Kisselgof, and T. R. Martin. 1999. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am. J. Respir. Crit. Care Med. 160:1150-1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

