AdpC is a Prevotella intermedia 17 leucine-rich repeat internalin-like protein
- PMID: 20308299
- PMCID: PMC2876575
- DOI: 10.1128/IAI.00510-09
AdpC is a Prevotella intermedia 17 leucine-rich repeat internalin-like protein
Abstract
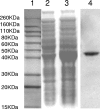
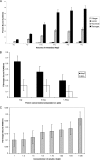
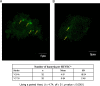
The oral bacterium Prevotella intermedia attaches to and invades gingival epithelial cells, fibroblasts, and endothelial cells. Several genes encoding proteins that mediate both the adhesion and invasion processes are carried on the genome of this bacterium. Here, we characterized one such protein, AdpC, belonging to the leucine-rich repeat (LRR) protein family. Bioinformatics analysis revealed that this protein shares similarity with the Treponema pallidum LRR (LRR(TP)) family of proteins and contains six LRRs. Despite the absence of a signal peptide, this protein is localized on the bacterial outer membrane, indicating that it is transported through an atypical secretion mechanism. The recombinant form of this protein (rAdpC) was shown to bind fibrinogen. In addition, the heterologous host strain Escherichia coli BL21 expressing rAdpC (V2846) invaded fibroblast NIH 3T3 cells at a 40-fold-higher frequency than control E. coli BL21 cells expressing a sham P. intermedia 17 protein. Although similar results were obtained by using human umbilical vein endothelial cells (HUVECs), only a 3-fold-increased invasion of V2846 into oral epithelial HN4 cells was observed. Thus, AdpC-mediated invasion is cell specific. This work demonstrated that AdpC is an important invasin protein of P. intermedia 17.
Figures


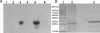



Similar articles
-
Interaction of Prevotella intermedia strain 17 leucine-rich repeat domain protein AdpF with eukaryotic cells promotes bacterial internalization.Infect Immun. 2014 Jun;82(6):2637-48. doi: 10.1128/IAI.01361-13. Epub 2014 Apr 7. Infect Immun. 2014. PMID: 24711565 Free PMC article.
-
Comparative genome analysis of Prevotella intermedia strain isolated from infected root canal reveals features related to pathogenicity and adaptation.BMC Genomics. 2015 Feb 25;16(1):122. doi: 10.1186/s12864-015-1272-3. BMC Genomics. 2015. PMID: 25765460 Free PMC article.
-
Characterization of the lactoferrin-dependent inhibition of the adhesion of Actinobacillus actinomycetemcomitans, Prevotella intermedia and Prevotella nigrescens to fibroblasts and to a reconstituted basement membrane.APMIS. 1997 Sep;105(9):680-8. doi: 10.1111/j.1699-0463.1997.tb05071.x. APMIS. 1997. PMID: 9350211
-
Surface antigens of the syphilis spirochete and their potential as virulence determinants.Emerg Infect Dis. 1997 Jan-Mar;3(1):11-20. doi: 10.3201/eid0301.970102. Emerg Infect Dis. 1997. PMID: 9126440 Free PMC article. Review.
-
Cloning and expression of Treponema pallidum protein antigens in Escherichia coli.DNA. 1982;1(4):329-33. doi: 10.1089/dna.1982.1.329. DNA. 1982. PMID: 6762960 Review.
Cited by
-
Interaction of Prevotella intermedia strain 17 leucine-rich repeat domain protein AdpF with eukaryotic cells promotes bacterial internalization.Infect Immun. 2014 Jun;82(6):2637-48. doi: 10.1128/IAI.01361-13. Epub 2014 Apr 7. Infect Immun. 2014. PMID: 24711565 Free PMC article.
-
C-terminal glycosylation of type IX secretion system cargo proteins in Prevotella intermedia with both short and long secretion signals.Open Biol. 2025 Mar;15(3):240335. doi: 10.1098/rsob.240335. Epub 2025 Mar 26. Open Biol. 2025. PMID: 40132644 Free PMC article.
-
The intracellular citrus huanglongbing bacterium, 'Candidatus Liberibacter asiaticus' encodes two novel autotransporters.PLoS One. 2013 Jul 11;8(7):e68921. doi: 10.1371/journal.pone.0068921. Print 2013. PLoS One. 2013. PMID: 23874813 Free PMC article.
-
Evaluation of Immunoprotective Effects of Fusobacterium necrophorum Outer Membrane Proteins 43K OMP, Leukotoxin and Hemolysin Multi-Component Recombinant Subunit Vaccine in Mice.Front Vet Sci. 2021 Dec 6;8:780377. doi: 10.3389/fvets.2021.780377. eCollection 2021. Front Vet Sci. 2021. PMID: 34938794 Free PMC article.
-
Tannerella forsythia invasion in oral epithelial cells requires phosphoinositide 3-kinase activation and clathrin-mediated endocytosis.Microbiology (Reading). 2011 Aug;157(Pt 8):2382-2391. doi: 10.1099/mic.0.048975-0. Epub 2011 May 26. Microbiology (Reading). 2011. PMID: 21622527 Free PMC article.
References
-
- Baumgartner, J. C., B. J. Watkins, K. S. Bae, and T. Xia. 1999. Association of black-pigmented bacteria with endodontic infections. J. Endod. 25:413-415. - PubMed
-
- Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, the European Listeria Gene Consortium, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. - PubMed
-
- Bierne, H., C. Sabet, N. Personnic, and P. Cossart. 2007. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect. 9:1156-1166. - PubMed
-
- Reference deleted.
-
- Braun, L., and P. Cossart. 2000. Interactions between Listeria monocytogenes and host mammalian cells. Microbes Infect. 2:803-811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

