Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties
- PMID: 20308300
- PMCID: PMC2876579
- DOI: 10.1128/IAI.01249-09
Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties
Abstract
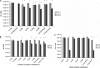
The HT29MTXE12 (E12) cell line harbors an adherent mucus layer, providing a novel technique to model mucosal infection in vitro. In this study, we have characterized the interaction of Campylobacter jejuni with the E12 cell line and exploited its unique mucus layer to examine the potential efficacy of probiotic treatment to attenuate C. jejuni virulence properties. C. jejuni 81-176 colonized and reproduced in E12 mucus. Adhesion to and internalization of C. jejuni were enhanced in E12 cells harboring mucus compared to parental cells without mucus. Translocation of C. jejuni occurred at early time points following infection. C. jejuni aligned with tight junctions and colocalized with the tight junction protein occludin, suggesting a paracellular route of translocation. Probiotic strains Lactobacillus rhamnosus R0011, Lactobacillus helveticus R0052, Lactobacillus salivarius AH102, Bifidobacterium longum AH1205, a commercial combination of L. rhamnosus R0011 and L. helveticus R0052 (Lacidofil), and a cocktail consisting of L. rhamnosus, L. helveticus, and L. salivarius (RhHeSa) colonized E12 mucus and bound to underlying cells. Probiotics attenuated C. jejuni association with and internalization into E12 cells and translocation to the basolateral medium of transwells. Live bacteria and prolonged precolonization of E12 cells with probiotics were necessary for probiotic action. These results demonstrate the potential for E12 cells as a model of mucosal pathogenesis and provide a rationale for the further investigation of probiotics as prophylaxis against human campylobacteriosis.
Figures


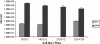



References
-
- Altenhoefer, A., S. Oswald, U. Sonnenborn, C. Enders, J. Schulze, J. Hacker, and T. A. Oelschlaeger. 2004. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40:223-229. - PubMed
-
- Barragan, A., F. Brossier, and L. D. Sibley. 2005. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 7:561-568. - PubMed
-
- Behrens, I., P. Stenberg, P. Artursson, and T. Kissel. 2001. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm. Res. 18:1138-1145. - PubMed
-
- Bras, A. M., and J. M. Ketley. 1999. Transcellular translocation of Campylobacter jejuni across human polarised epithelial monolayers. FEMS Microbiol. Lett. 179:209-215. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

