Inflammatory cytokine response to Bacillus anthracis peptidoglycan requires phagocytosis and lysosomal trafficking
- PMID: 20308305
- PMCID: PMC2876538
- DOI: 10.1128/IAI.00170-10
Inflammatory cytokine response to Bacillus anthracis peptidoglycan requires phagocytosis and lysosomal trafficking
Abstract
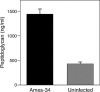
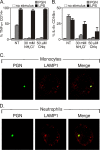
During advanced stages of inhalation anthrax, Bacillus anthracis accumulates at high levels in the bloodstream of the infected host. This bacteremia leads to sepsis during late-stage anthrax; however, the mechanisms through which B. anthracis-derived factors contribute to the pathology of infected hosts are poorly defined. Peptidoglycan, a major component of the cell wall of Gram-positive bacteria, can provoke symptoms of sepsis in animal models. We have previously shown that peptidoglycan of B. anthracis can induce the production of proinflammatory cytokines by cells in human blood. Here, we show that biologically active peptidoglycan is shed from an active culture of encapsulated B. anthracis strain Ames in blood. Peptidoglycan is able to bind to surfaces of responding cells, and internalization of peptidoglycan is required for the production of inflammatory cytokines. We also show that the peptidoglycan traffics to lysosomes, and lysosomal function is required for cytokine production. We conclude that peptidoglycan of B. anthracis is initially bound by an unknown extracellular receptor, is phagocytosed, and traffics to lysosomes, where it is degraded to a product recognized by an intracellular receptor. Binding of the peptidoglycan product to the intracellular receptor causes a proinflammatory response. These findings provide new insight into the mechanism by which B. anthracis triggers sepsis during a critical stage of anthrax disease.
Figures




References
-
- Baldari, C. T., F. Tonello, S. R. Paccani, and C. Montecucco. 2006. Anthrax toxins: a paradigm of bacterial immune suppression. Trends Immunol. 27:434-440. - PubMed
-
- Biswas, S. K., and E. Lopez-Collazo. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30:475-487. - PubMed
-
- Chaloupka, J., and P. Kreckova. 1971. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol. (Praha) 16:372-382. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. - PubMed
-
- Chau, T. A., M. L. McCully, W. Brintnell, G. An, K. J. Kasper, E. D. Vines, P. Kubes, S. M. Haeryfar, J. K. McCormick, E. Cairns, D. E. Heinrichs, and J. Madrenas. 2009. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 15:641-648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

