Antagonism of the complement component C4 by flavivirus nonstructural protein NS1
- PMID: 20308361
- PMCID: PMC2856034
- DOI: 10.1084/jem.20092545
Antagonism of the complement component C4 by flavivirus nonstructural protein NS1
Abstract
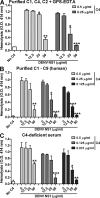
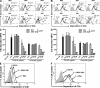
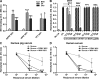
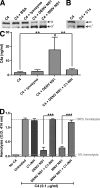
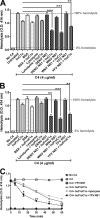
The complement system plays an essential protective role in the initial defense against many microorganisms. Flavivirus NS1 is a secreted nonstructural glycoprotein that accumulates in blood, is displayed on the surface of infected cells, and has been hypothesized to have immune evasion functions. Herein, we demonstrate that dengue virus (DENV), West Nile virus (WNV), and yellow fever virus (YFV) NS1 attenuate classical and lectin pathway activation by directly interacting with C4. Binding of NS1 to C4 reduced C4b deposition and C3 convertase (C4b2a) activity. Although NS1 bound C4b, it lacked intrinsic cofactor activity to degrade C4b, and did not block C3 convertase formation or accelerate decay of the C3 and C5 convertases. Instead, NS1 enhanced C4 cleavage by recruiting and activating the complement-specific protease C1s. By binding C1s and C4 in a complex, NS1 promotes efficient degradation of C4 to C4b. Through this mechanism, NS1 protects DENV from complement-dependent neutralization in solution. These studies define a novel immune evasion mechanism for restricting complement control of microbial infection.
Figures



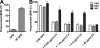
References
-
- Alcon S., Talarmin A., Debruyne M., Falconar A., Deubel V., Flamand M. 2002. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376–381 10.1128/JCM.40.02.376-381.2002 - DOI - PMC - PubMed
-
- Alcon-LePoder S., Drouet M.T., Roux P., Frenkiel M.P., Arborio M., Durand-Schneider A.M., Maurice M., Le Blanc I., Gruenberg J., Flamand M. 2005. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J. Virol. 79:11403–11411 10.1128/JVI.79.17.11403-11411.2005 - DOI - PMC - PubMed
-
- Avirutnan P., Punyadee N., Noisakran S., Komoltri C., Thiemmeca S., Auethavornanan K., Jairungsri A., Kanlaya R., Tangthawornchaikul N., Puttikhunt C., et al. 2006. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 193:1078–1088 10.1086/500949 - DOI - PubMed
-
- Avirutnan P., Zhang L., Punyadee N., Manuyakorn A., Puttikhunt C., Kasinrerk W., Malasit P., Atkinson J.P., Diamond M.S. 2007. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 3:e183 10.1371/journal.ppat.0030183 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

