Population pharmacokinetics of micafungin in neonates and young infants
- PMID: 20308367
- PMCID: PMC2876406
- DOI: 10.1128/AAC.01679-09
Population pharmacokinetics of micafungin in neonates and young infants
Abstract
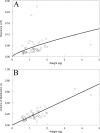
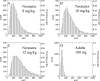
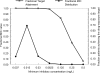
Micafungin is an echinocandin with potent activity against Candida spp. Hematogenous Candida meningoencephalitis (HCME) is a frequent complication of disseminated Candida infection in premature infants. A preclinical model of HCME suggests that micafungin may be an effective agent for this syndrome, but relatively high weight-based dosages are required. This prompted the further study of the safety and pharmacokinetics (PK) of micafungin in infants. Here, we describe the population pharmacokinetics of micafungin in 47 infants with a proven or presumptive diagnosis of disseminated candidiasis, who received 0.75, 1.5, 3, 7, 10, and 15 mg/kg of micafungin. The drug was infused daily, and samples were taken in the first dosing interval and at steady state. Serum concentrations were measured using high-performance liquid chromatography (HPLC). Data were modeled using an allometric pharmacokinetic model using a three-fourths scaling exponent for clearance and parameters normalized to a 70-kg adult. Drug exposures were estimated using Monte Carlo simulation. Optimal sampling times were determined using D-optimal design theory. The fit of the allometric model to the data was highly acceptable. The pharmacokinetics of micafungin were linear. The weight-normalized estimates of clearance and volume of distribution approximated those previously described for adults. The original population parameter values could be recapitulated in the Monte Carlo simulations. A dosage of 10 mg/kg/day resulted in 82.6% of patients with areas under the concentration-time curve (AUCs) that are associated with near-maximal decline in fungal burden within the central nervous system (CNS).
Figures

Similar articles
-
The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates.J Infect Dis. 2008 Jan 1;197(1):163-71. doi: 10.1086/524063. J Infect Dis. 2008. PMID: 18171300 Free PMC article.
-
Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients.Clin Pharmacokinet. 2017 Oct;56(10):1197-1206. doi: 10.1007/s40262-017-0509-5. Clin Pharmacokinet. 2017. PMID: 28144840 Free PMC article.
-
Pharmacokinetics and Safety of Micafungin in Infants Supported With Extracorporeal Membrane Oxygenation.Pediatr Infect Dis J. 2016 Nov;35(11):1204-1210. doi: 10.1097/INF.0000000000001268. Pediatr Infect Dis J. 2016. PMID: 27314826 Free PMC article.
-
[Why might micafungin be the drug of choice in pediatric patients?].Enferm Infecc Microbiol Clin. 2011 Mar;29 Suppl 2:23-8. doi: 10.1016/S0213-005X(11)70005-2. Enferm Infecc Microbiol Clin. 2011. PMID: 21420573 Review. Spanish.
-
The management of Candida infections in preterm neonates and the role of micafungin.J Matern Fetal Neonatal Med. 2011 Oct;24 Suppl 2:24-7. doi: 10.3109/14767058.2011.604929. J Matern Fetal Neonatal Med. 2011. PMID: 21749182 Review.
Cited by
-
Perspectives on the Use of Echinocandins in the Neonatal Intensive Care Unit.Antibiotics (Basel). 2024 Dec 12;13(12):1209. doi: 10.3390/antibiotics13121209. Antibiotics (Basel). 2024. PMID: 39766599 Free PMC article. Review.
-
Antifungal agents and therapy for infants and children with invasive fungal infections: a pharmacological perspective.Br J Clin Pharmacol. 2013 Jun;75(6):1381-95. doi: 10.1111/bcp.12025. Br J Clin Pharmacol. 2013. PMID: 23126319 Free PMC article. Review.
-
Population pharmacokinetics of cyclosporine in Chinese children receiving hematopoietic stem cell transplantation.Acta Pharmacol Sin. 2019 Dec;40(12):1603-1610. doi: 10.1038/s41401-019-0277-x. Epub 2019 Jul 24. Acta Pharmacol Sin. 2019. PMID: 31341257 Free PMC article.
-
Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology.Adv Drug Deliv Rev. 2014 Jun;73(100):127-39. doi: 10.1016/j.addr.2014.01.002. Epub 2014 Jan 17. Adv Drug Deliv Rev. 2014. PMID: 24440429 Free PMC article. Review.
-
Considerations in the pharmacologic treatment and prevention of neonatal sepsis.Paediatr Drugs. 2014 Feb;16(1):67-81. doi: 10.1007/s40272-013-0057-x. Paediatr Drugs. 2014. PMID: 24218112 Review.
References
-
- Benjamin, D. K., Jr., C. Poole, W. J. Steinbach, J. L. Rowen, and T. J. Walsh. 2003. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics 112:634-640. - PubMed
-
- Benjamin, D. K., Jr., B. J. Stoll, A. A. Fanaroff, S. A. McDonald, W. Oh, R. D. Higgins, S. Duara, K. Poole, A. Laptook, and R. Goldberg. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84-92. - PubMed
-
- D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA. http://bmsr.esc.edu/.
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- 1R01HD057956-02/HD/NICHD NIH HHS/United States
- U10 HD045962/HD/NICHD NIH HHS/United States
- 1K24HD058735-01/HD/NICHD NIH HHS/United States
- R01 FD003519/FD/FDA HHS/United States
- 1R01FD003519-01/FD/FDA HHS/United States
- K23 HD060040/HD/NICHD NIH HHS/United States
- 1U10HD45962-06/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- 1K23HD060040-01/HD/NICHD NIH HHS/United States
- 1U10-HD45962-06/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

