Preclinical in vitro and in vivo characterization of the fully human monoclonal IgM antibody KBPA101 specific for Pseudomonas aeruginosa serotype IATS-O11
- PMID: 20308370
- PMCID: PMC2876355
- DOI: 10.1128/AAC.01142-09
Preclinical in vitro and in vivo characterization of the fully human monoclonal IgM antibody KBPA101 specific for Pseudomonas aeruginosa serotype IATS-O11
Abstract
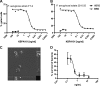
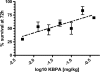
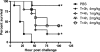
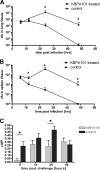
Pseudomonas aeruginosa infection in ventilator-associated pneumonia is a serious and often life-threatening complication in intensive care unit patients, and new treatment options are needed. We used B-cell-enriched peripheral blood lymphocytes from a volunteer immunized with a P. aeruginosa O-polysaccharide-toxin A conjugate vaccine to generate human hybridoma cell lines producing monoclonal antibodies specific for individual P. aeruginosa lipopolysaccharide serotypes. The fully human monoclonal antibody secreted by one of these lines, KBPA101, is an IgM/kappa antibody that binds P. aeruginosa of International Antigenic Typing System (IATS) serotype O11 with high avidity (5.81 x 10(7) M(-1) +/- 2.8 x 10(7) M(-1)) without cross-reacting with other serotypes. KBPA101 specifically opsonized the P. aeruginosa of IATS O11 serotype and mediated complement-dependent phagocytosis in vitro by the human monocyte-like cell line HL-60 at a very low concentration (half-maximal phagocytosis at 0.16 ng/ml). In vivo evaluation of KBPA101 demonstrated a dose-response relationship for protection against systemic infections in a murine burn wound sepsis model, where 70 to 100% of animals were protected against lethal challenges with P. aeruginosa at doses as low as 5 microg/animal. Furthermore, a high efficacy of KBPA101 in protection from local respiratory infections in an acute lung infection model in mice was demonstrated. Preclinical toxicology evaluation on human tissue, in rabbits, and in mice did not indicate any toxicity of KBPA101. Based on these preclinical findings, the first human clinical trials have been initiated.
Figures

Similar articles
-
Opsonic and protective activity of five human IgM monoclonal antibodies reactive with lipopolysaccharide antigen of Pseudomonas aeruginosa.FEMS Microbiol Immunol. 1990 Dec;2(5-6):263-8. doi: 10.1111/j.1574-6968.1990.tb03528.x. FEMS Microbiol Immunol. 1990. PMID: 2127368
-
Anti-Pseudomonas aeruginosa serotype O11 LPS immunoglobulin M monoclonal antibody panobacumab (KBPA101) confers protection in a murine model of acute lung infection.J Antimicrob Chemother. 2011 May;66(5):1100-9. doi: 10.1093/jac/dkr038. Epub 2011 Feb 24. J Antimicrob Chemother. 2011. PMID: 21393169
-
A comparative characterization of dipentameric (IgM)(2) and pentameric IgM species present in preparations of a monoclonal IgM for therapeutic use.J Pharm Biomed Anal. 2010 Apr 6;51(5):1084-90. doi: 10.1016/j.jpba.2009.11.003. Epub 2009 Nov 13. J Pharm Biomed Anal. 2010. PMID: 20005064
-
Vaccines and immunotherapy against Pseudomonas aeruginosa.Vaccine. 2008 Feb 20;26(8):1011-24. doi: 10.1016/j.vaccine.2007.12.007. Epub 2007 Dec 26. Vaccine. 2008. PMID: 18242792 Review.
-
Pseudomonas aeruginosa antigens as potential vaccines.FEMS Microbiol Rev. 1997 Nov;21(3):243-77. doi: 10.1111/j.1574-6976.1997.tb00353.x. FEMS Microbiol Rev. 1997. PMID: 9451816 Review.
Cited by
-
Recent developments for Pseudomonas vaccines.Hum Vaccin. 2011 Oct;7(10):999-1011. doi: 10.4161/hv.7.10.16369. Epub 2011 Oct 1. Hum Vaccin. 2011. PMID: 21941090 Free PMC article. Review.
-
A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models.Antimicrob Agents Chemother. 2014 Aug;58(8):4384-91. doi: 10.1128/AAC.02643-14. Epub 2014 May 19. Antimicrob Agents Chemother. 2014. PMID: 24841258 Free PMC article.
-
Pangenome-wide and molecular evolution analyses of the Pseudomonas aeruginosa species.BMC Genomics. 2016 Jan 12;17:45. doi: 10.1186/s12864-016-2364-4. BMC Genomics. 2016. PMID: 26754847 Free PMC article.
-
Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia.Eur J Clin Microbiol Infect Dis. 2014 Oct;33(10):1861-7. doi: 10.1007/s10096-014-2156-1. Epub 2014 May 24. Eur J Clin Microbiol Infect Dis. 2014. PMID: 24859907 Clinical Trial.
-
Expression and glycoengineering of functionally active heteromultimeric IgM in plants.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6263-8. doi: 10.1073/pnas.1320544111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706782 Free PMC article.
References
-
- Bruderer, U., S. J. Cryz, Jr., U. B. Schaad, M. Deusinger, J. U. Que, and A. B. Lang. 1992. Affinity constants of naturally acquired and vaccine-induced anti-Pseudomonas aeruginosa antibodies in healthy adults and cystic fibrosis patients. J. Infect. Dis. 166:344-349. - PubMed
-
- Casadevall, A., E. Dadachova, and L. A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695-703. - PubMed
-
- Crouch Brewer, S., R. G. Wunderink, C. B. Jones, and K. V. Leeper, Jr. 1996. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109:1019-1029. - PubMed
-
- Cryz, S. J., Jr., A. Lang, A. Rudeberg, J. Wedgwood, J. U. Que, E. Furer, and U. Schaad. 1997. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst. Mitt:345-349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

