Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation
- PMID: 20308429
- PMCID: PMC2845065
- DOI: 10.1083/jcb.200906012
Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation
Abstract
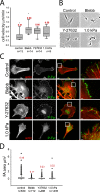
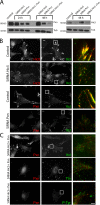
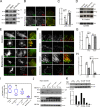
Focal adhesions (FAs) are mechanosensitive adhesion and signaling complexes that grow and change composition in response to myosin II-mediated cytoskeletal tension in a process known as FA maturation. To understand tension-mediated FA maturation, we sought to identify proteins that are recruited to FAs in a myosin II-dependent manner and to examine the mechanism for their myosin II-sensitive FA association. We find that FA recruitment of both the cytoskeletal adapter protein vinculin and the tyrosine kinase FA kinase (FAK) are myosin II and extracellular matrix (ECM) stiffness dependent. Myosin II activity promotes FAK/Src-mediated phosphorylation of paxillin on tyrosines 31 and 118 and vinculin association with paxillin. We show that phosphomimic mutations of paxillin can specifically induce the recruitment of vinculin to adhesions independent of myosin II activity. These results reveal an important role for paxillin in adhesion mechanosensing via myosin II-mediated FAK phosphorylation of paxillin that promotes vinculin FA recruitment to reinforce the cytoskeletal ECM linkage and drive FA maturation.
Figures



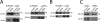

References
-
- Brown M.C., Perrotta J.A., Turner C.E. 1996. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135:1109–1123 10.1083/jcb.135.4.1109 - DOI - PMC - PubMed
-
- Bulinski J.C., Odde D.J., Howell B.J., Salmon T.D., Waterman-Storer C.M. 2001. Rapid dynamics of the microtubule binding of ensconsin in vivo. J. Cell Sci. 114:3885–3897 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

