Regulation of androgen-responsive transcription by the chromatin remodeling factor CHD8
- PMID: 20308527
- PMCID: PMC2875808
- DOI: 10.1210/me.2009-0421
Regulation of androgen-responsive transcription by the chromatin remodeling factor CHD8
Abstract
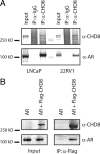
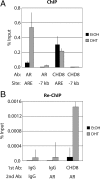
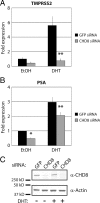
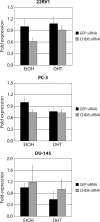
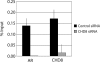
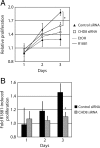
The androgen receptor (AR) mediates the effect of androgens through its transcriptional function during both normal prostate development and in the emergence and progression of prostate cancer. AR is known to assemble coactivator complexes at target promoters to facilitate transcriptional activation in response to androgens. Here we identify the ATP-dependent chromatin remodeling factor chromodomain helicase DNA-binding protein 8 (CHD8) as a novel coregulator of androgen-responsive transcription. We demonstrate that CHD8 directly associates with AR and that CHD8 and AR simultaneously localize to the TMPRSS2 enhancer after androgen treatment. In the LNCaP cell line, reduction of CHD8 levels by small interfering RNA treatment severely diminishes androgen-dependent activation of the TMPRSS2 gene. We demonstrate that the recruitment of AR to the TMPRSS2 promoter in response to androgen treatment requires CHD8. Finally, CHD8 facilitates androgen-stimulated proliferation of LNCaP cells, emphasizing the physiological importance of CHD8. Taken together, we present evidence of a functional role for CHD8 in AR-mediated transcriptional regulation of target genes.
Figures
Similar articles
-
The Deubiquitinating Enzyme USP7 Regulates Androgen Receptor Activity by Modulating Its Binding to Chromatin.J Biol Chem. 2015 Aug 28;290(35):21713-23. doi: 10.1074/jbc.M114.628255. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175158 Free PMC article.
-
Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation.Proc Natl Acad Sci U S A. 2007 May 15;104(20):8438-43. doi: 10.1073/pnas.0700420104. Epub 2007 May 9. Proc Natl Acad Sci U S A. 2007. PMID: 17494760 Free PMC article.
-
Regulation of protein kinase C-related kinase (PRK) signalling by the TPα and TPβ isoforms of the human thromboxane A2 receptor: Implications for thromboxane- and androgen- dependent neoplastic and epigenetic responses in prostate cancer.Biochim Biophys Acta Mol Basis Dis. 2017 Apr;1863(4):838-856. doi: 10.1016/j.bbadis.2017.01.011. Epub 2017 Jan 18. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28108419
-
The Mechanisms of CHD8 in Neurodevelopment and Autism Spectrum Disorders.Genes (Basel). 2021 Jul 26;12(8):1133. doi: 10.3390/genes12081133. Genes (Basel). 2021. PMID: 34440307 Free PMC article. Review.
-
From X-inactivation to neurodevelopment: CHD8-transcription factors (TFs) competitive binding at regulatory regions of CHD8 target genes can contribute to correct neuronal differentiation.Curr Res Neurobiol. 2023 Nov 7;5:100114. doi: 10.1016/j.crneur.2023.100114. eCollection 2023. Curr Res Neurobiol. 2023. PMID: 38020809 Free PMC article. Review.
Cited by
-
Histone H1 recruitment by CHD8 is essential for suppression of the Wnt-β-catenin signaling pathway.Mol Cell Biol. 2012 Jan;32(2):501-12. doi: 10.1128/MCB.06409-11. Epub 2011 Nov 14. Mol Cell Biol. 2012. PMID: 22083958 Free PMC article.
-
The Chromodomain Helicase DNA-Binding Chromatin Remodelers: Family Traits that Protect from and Promote Cancer.Cold Spring Harb Perspect Med. 2017 Apr 3;7(4):a026450. doi: 10.1101/cshperspect.a026450. Cold Spring Harb Perspect Med. 2017. PMID: 28096241 Free PMC article. Review.
-
High glucose-ROS conditions enhance the progression in cholangiocarcinoma via upregulation of MAN2A2 and CHD8.Cancer Sci. 2021 Jan;112(1):254-264. doi: 10.1111/cas.14719. Epub 2020 Nov 29. Cancer Sci. 2021. PMID: 33141432 Free PMC article.
-
Non-Coding RNAs and Nucleosome Remodeling Complexes: An Intricate Regulatory Relationship.Biology (Basel). 2020 Aug 7;9(8):213. doi: 10.3390/biology9080213. Biology (Basel). 2020. PMID: 32784701 Free PMC article. Review.
-
Chromatin remodelers: We are the drivers!!Nucleus. 2016 Jul 3;7(4):388-404. doi: 10.1080/19491034.2016.1211217. Epub 2016 Jul 18. Nucleus. 2016. PMID: 27429206 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ 2009 Cancer statistics, 2009. CA Cancer J Clin 59:225–249 - PubMed
-
- Dehm SM, Tindall DJ 2006 Molecular regulation of androgen action in prostate cancer. J Cell Biochem 99:333–344 - PubMed
-
- Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Furasato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul JW, Srivastava S 2005 Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24:3847–3852 - PubMed
-
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 - PubMed
-
- Denis LJ, Griffiths K 2000 Endocrine treatment in prostate cancer. Semin Surg Oncol 18:52–74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

