The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary
- PMID: 20308529
- PMCID: PMC2875814
- DOI: 10.1210/en.2010-0081
The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary
Abstract
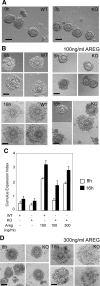
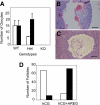
The nuclear receptor cofactor receptor-interacting protein 140 (RIP140) is essential for cumulus cell-oocyte complex (COC) expansion, follicular rupture, and oocyte release during ovulation. The expression of many genes necessary for COC expansion is impaired in the absence of RIP140, but the studies herein document that their expression can be restored and COC expansion rescued by treatment with the epidermal growth factor (EGF)-like factor amphiregulin (AREG) both in vitro and in vivo. We demonstrate by several approaches that RIP140 is required for the expression of the EGF-like factors in granulosa cells, but the dependence of genes involved in cumulus expansion, including Ptgs2 Has2, Tnfaip6, and Ptx3, is indirect because they are induced by AREG. Treatment of granulosa cells with forskolin to mimic the effects of LH increases AREG promoter activity in a RIP140-dependent manner that 1) requires an intact cAMP response element in the proximal promoter region of the Areg gene and 2) involves its actions as a coactivator for cAMP response element-binding protein/c-Jun transcription factors. Although human chorionic gonadotropin and AREG coadministration is sufficient to restore ovulation fully in RIP140 heterozygous mice in vivo, both follicular rupture and ovulation remain impaired in the RIP140 null mice. Thus, we conclude that although the level of RIP140 expression in the ovary is a crucial factor required for the transient expression of EGF-like factors necessary for cumulus expansion, it also plays a role in other signaling pathways that induce follicular rupture.
Figures




References
-
- Richards JS 2005 Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 - PubMed
-
- Richards JS 2007 Genetics of ovulation. Semin Reprod Med 25:235–242 - PubMed
-
- Russell DL, Robker RL 2007 Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 13:289–312 - PubMed
-
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

