Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins
- PMID: 20308533
- PMCID: PMC2869266
- DOI: 10.1210/en.2009-1227
Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins
Abstract
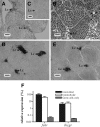
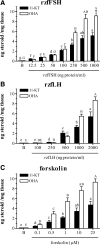
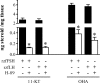
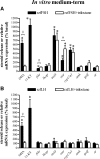
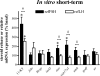
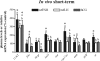
This study aimed to improve, using the zebrafish model, our understanding of the distinct roles of pituitary gonadotropins FSH and LH in regulating testis functions in teleost fish. We report, for the first time in a vertebrate species, that zebrafish Leydig cells as well as Sertoli cells express the mRNAs for both gonadotropin receptors (fshr and lhcgr). Although Leydig cell fshr expression has been reported in other piscine species and may be a common feature of teleost fish, Sertoli cell lhcgr expression has not been reported previously and might be related to the undifferentiated gonochoristic mode of gonadal sex differentiation in zebrafish. Both recombinant zebrafish (rzf) gonadotropins (i.e. rzfLH and rzfFSH) stimulated androgen release in vitro and in vivo, with rzfFSH being significantly more potent than rzfLH. Forskolin-induced adenylate cyclase activation mimicked, whereas the protein kinase A inhibitor H-89 significantly reduced, the gonadotropin-stimulated androgen release. Therefore, we conclude that both FSH receptor and LH/choriogonadotropin receptor signaling are predominantly mediated through the cAMP/protein kinase A pathway to promote steroid production. Despite this similarity, other downstream mechanisms seem to differ. For example, rzfFSH up-regulated the testicular mRNA levels of a number of steroidogenesis-related genes both in vitro and in vivo, whereas rzfLH or human chorionic gonadotropin did not. Although not fully understood at present, these differences could explain the capacity of FSH to support both steroidogenesis and spermatogenesis on a long-term basis, whereas LH-stimulated steroidogenesis might be a more acute process, possibly restricted to periods during which peak steroid levels are required.
Figures
References
-
- Pierce JG, Parsons TF 1981 Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495 - PubMed
-
- McLachlan RI, Wreford NG, O'Donnell L, de Kretser DM, Robertson DM 1996 The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol 148:1–9 - PubMed
-
- Kumar TR 2005 What have we learned about gonadotropin function from gonadotropin subunit and receptor knockout mice? Reproduction 130:293–302 - PubMed
-
- Petersen C, Soder O 2006 The Sertoli cell: a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 66:153–161 - PubMed
-
- Saez JM 1994 Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev 15:574–626 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

