Coordination of Rab8 and Rab11 in primary ciliogenesis
- PMID: 20308558
- PMCID: PMC2851980
- DOI: 10.1073/pnas.1002401107
Coordination of Rab8 and Rab11 in primary ciliogenesis
Abstract
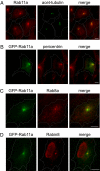
Primary cilia are microtubule-based membrane projections located at the surface of many cells. Defects in primary cilia formation have been implicated in a number of genetic disorders, such as Bardet-Biedl Syndrome and Polycystic Kidney Disease. Recent studies have demonstrated that polarized vesicular transport involving Rab8 and its guanine nucleotide-exchange factor Rabin8 is essential for primary ciliogenesis. Here we report that Rabin8 is a direct downstream effector of Rab11, which functions in membrane trafficking from the trans-Golgi network and recycling endosomes. Rab11, in its GTP-bound form, interacts with Rabin8 and kinetically stimulates the guanine nucleotide-exchange activity of Rabin8 toward Rab8. Rab11 is enriched at the base of the primary cilia and inhibition of Rab11 function by a dominant-negative mutant or RNA interference blocks primary ciliogenesis. Our results suggest that Rab GTPases coordinate with each other in the regulation of vesicular trafficking during primary ciliogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. - PubMed
-
- Leroux MR. Taking vesicular transport to the cilium. Cell. 2007;129:1041–1043. - PubMed
-
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

