P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis
- PMID: 20308559
- PMCID: PMC2851979
- DOI: 10.1073/pnas.0911082107
P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis
Abstract
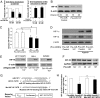
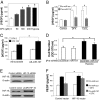
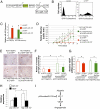
The pathway involving the tumor suppressor gene TP53 can regulate tumor angiogenesis by unclear mechanisms. Here we show that p53 regulates hypoxic signaling through the transcriptional regulation of microRNA-107 (miR-107). We found that miR-107 is a microRNA expressed by human colon cancer specimens and regulated by p53. miR-107 decreases hypoxia signaling by suppressing expression of hypoxia inducible factor-1beta (HIF-1beta). Knockdown of endogenous miR-107 enhances HIF-1beta expression and hypoxic signaling in human colon cancer cells. Conversely, overexpression of miR-107 inhibits HIF-1beta expression and hypoxic signaling. Furthermore, overexpression of miR-107 in tumor cells suppresses tumor angiogenesis, tumor growth, and tumor VEGF expression in mice. Finally, in human colon cancer specimens, expression of miR-107 is inversely associated with expression of HIF-1beta. Taken together these data suggest that miR-107 can mediate p53 regulation of hypoxic signaling and tumor angiogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
-
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. - PubMed
-
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. - PubMed
-
- Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8:269–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

