Association of RIG-I with innate immunity of ducks to influenza
- PMID: 20308570
- PMCID: PMC2851864
- DOI: 10.1073/pnas.1001755107
Association of RIG-I with innate immunity of ducks to influenza
Erratum in
- Proc Natl Acad Sci U S A. 2013 May 7;110(19):7958
Abstract
Ducks and wild waterfowl perpetuate all strains of influenza viruses in nature. In their natural host, influenza viruses typically cause asymptomatic infection and little pathology. Ducks are often resistant to influenza viruses capable of killing chickens. Here, we show that the influenza virus sensor, RIG-I, is present in ducks and plays a role in clearing an influenza infection. We show evidence suggesting that RIG-I may be absent in chickens, providing a plausible explanation for their increased susceptibility to influenza viruses compared with ducks. RIG-I detects RNA ligands derived from uncapped viral transcripts and initiates the IFN response. In this study, we show that the chicken embryonic fibroblast cell line, DF-1, cannot respond to a RIG-I ligand. However, transfection of duck RIG-I into DF-1 cells rescues the detection of ligand and induces IFN-beta promoter activity. Additionally, DF-1 cells expressing duck RIG-I have an augmented IFN response resulting in decreased influenza replication after challenge with either low or highly pathogenic avian influenza virus. Implicating RIG-I in the antiviral response to an infection in vivo, we found that RIG-I expression is induced 200 fold, early in an innate immune response in ducks challenged with the H5N1 virus A/Vietnam/1203/04. Finding this natural disease resistance gene in ducks opens the possibility of increasing influenza resistance through creation of a transgenic chicken.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

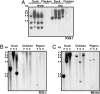
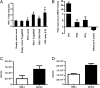
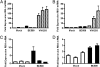
References
-
- World Health Organization Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to the World Health Organization. 2009. Available at http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_.... Accessed December 16, 2009.
-
- Ellis TM, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

