Ribosome-associated peroxiredoxins suppress oxidative stress-induced de novo formation of the [PSI+] prion in yeast
- PMID: 20308573
- PMCID: PMC2851942
- DOI: 10.1073/pnas.1000347107
Ribosome-associated peroxiredoxins suppress oxidative stress-induced de novo formation of the [PSI+] prion in yeast
Abstract
Peroxiredoxins (Prxs) are ubiquitous antioxidants that protect cells against oxidative stress. We show that the yeast Tsa1/Tsa2 Prxs colocalize to ribosomes and function to protect the Sup35 translation termination factor against oxidative stress-induced formation of its heritable [PSI(+)] prion conformation. In a tsa1 tsa2 [psi(-)] [PIN(+)] strain, the frequency of [PSI(+)] de novo formation is significantly elevated. The Tsa1/Tsa2 Prxs, like other 2-Cys Prxs, have dual activities as peroxidases and chaperones, and we show that the peroxidase activity is required to suppress spontaneous de novo [PSI(+)] prion formation. Molecular oxygen is required for [PSI(+)] prion formation as growth under anaerobic conditions prevents prion formation in the tsa1 tsa2 mutant. Conversely, oxidative stress conditions induced by exposure to hydrogen peroxide elevates the rate of de novo [PSI(+)] prion formation leading to increased suppression of all three termination codons in the tsa1 tsa2 mutant. Altered translational fidelity in [PSI(+)] strains may provide a mechanism that promotes genetic variation and phenotypic diversity (True HL, Lindquist SL (2000) Nature 407:477-483). In agreement, we find that prion formation provides yeast cells with an adaptive advantage under oxidative stress conditions, as elimination of the [PSI(+)] prion from tsa1 tsa2 mutants renders the resulting [psi(-)] [pin(-)] cells hypersensitive to hydrogen peroxide. These data support a model in which Prxs function to protect the ribosomal machinery against oxidative damage, but when these systems become overwhelmed, [PSI(+)] prion formation provides a mechanism for uncovering genetic traits that aid survival during oxidative stress conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

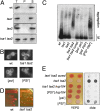

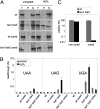
References
-
- Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. - PubMed
-
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. - PubMed
-
- Garrido EO, Grant CM. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol Microbiol. 2002;43:993–1003. - PubMed
-
- Wong CM, Siu KL, Jin DY. Peroxiredoxin-null yeast cells are hypersensitive to oxidative stress and are genomically unstable. J Biol Chem. 2004;279:23207–23213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

