Subunit interactions in bovine papillomavirus
- PMID: 20308582
- PMCID: PMC2852008
- DOI: 10.1073/pnas.0914604107
Subunit interactions in bovine papillomavirus
Abstract
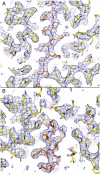
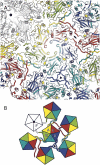
Papillomaviruses, members of a group of dsDNA viruses associated with epithelial growths and tumors, have compact capsids assembled from 72 pentamers of the protein L1. We have determined the structure of bovine papillomavirus by electron cryomicrosopy (cryoEM), at approximately 3.6 A resolution. The density map, obtained from single-particle analysis of approximately 4,000 particle images, shows the trace of the L1 polypeptide chain and reveals how the N- and C-terminal "arms" of a subunit (extensions from its beta-jelly-roll core) associate with a neighboring pentamer. Critical contacts come from the C-terminal arm, which loops out from the core of the subunit, forms contacts (including a disulfide) with two subunits in a neighboring pentamer, and reinserts into the pentamer from which it emanates. This trace corrects one feature of an earlier model. We discuss implications of the structure for virion assembly and for pathways of infectious viral entry. We suggest that it should be possible to obtain image reconstructions of comparable resolution from cryoEM images of asymmetric particles. From the work on papillomavirus described here, we estimate that such a reconstruction will require about 1.5 million images to achieve the same number of averaged asymmetric units; structural variability will increase this number substantially.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Howley PM, Lowy DR. Papillomaviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 2299–2354.
-
- Shi L, et al. GARDASIL: Prophylactic human papillomavirus vaccine development—From bench top to bed-side. Clin Pharmacol Ther. 2007;81:259–264. - PubMed
-
- Liddington RC, et al. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354:278–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

