Prion-like transmission of protein aggregates in neurodegenerative diseases
- PMID: 20308987
- PMCID: PMC2892479
- DOI: 10.1038/nrm2873
Prion-like transmission of protein aggregates in neurodegenerative diseases
Abstract
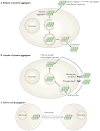
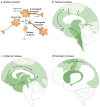
Neurodegenerative diseases are commonly associated with the accumulation of intracellular or extracellular protein aggregates. Recent studies suggest that these aggregates are capable of crossing cellular membranes and can directly contribute to the propagation of neurodegenerative disease pathogenesis. We propose that, once initiated, neuropathological changes might spread in a 'prion-like' manner and that disease progression is associated with the intercellular transfer of pathogenic proteins. The transfer of naked infectious particles between cells could therefore be a target for new disease-modifying therapies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
-
- Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem. 2007;103:17–37. - PubMed
-
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. - PubMed
-
- Braak H, et al. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

