Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides
- PMID: 20309487
- PMCID: PMC3073167
- DOI: 10.1039/b904091a
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides
Abstract
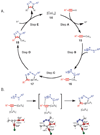
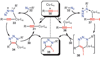
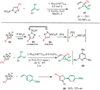
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) is a widely utilized, reliable, and straightforward way for making covalent connections between building blocks containing various functional groups. It has been used in organic synthesis, medicinal chemistry, surface and polymer chemistry, and bioconjugation applications. Despite the apparent simplicity of the reaction, its mechanism involves multiple reversible steps involving coordination complexes of copper(I) acetylides of varying nuclearity. Understanding and controlling these equilibria is of paramount importance for channeling the reaction into the productive catalytic cycle. This tutorial review examines the history of the development of the CuAAC reaction, its key mechanistic aspects, and highlights the features that make it useful to practitioners in different fields of chemical science.
Figures




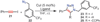
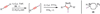

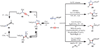
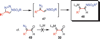
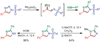
References
-
- Kolb HC, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004–2021. - PubMed
-
- Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3062. - PubMed
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:2596–2599. - PubMed
-
- Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med. Res. Rev. 2008;28:278–308. - PubMed
- Lutz J-F, Zarafshani Z. Adv. Drug Delivery Rev. 2008;60:958–970. - PubMed
- Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. - PubMed
- Fokin VV. ACS Chem. Biol. 2007;2:775–778. - PubMed
- Johnson JA, Koberstein JT, Finn MG, Turro NJ. Macromol. Rapid Commun. 2008;29:1052–1072.
- Bock VD, Hiemstra H, van Maarseveen JH. Eur. J. Org. Chem. 2006:51–68.
- Wu P, Fokin VV. Aldrichimica Acta. 2007;40:7–17.
-
- Meldal M, Tornoe CW. Chem. Rev. Vol. 108. Washington, DC, U. S.: 2008. pp. 2952–3015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

