Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice
- PMID: 20309866
- PMCID: PMC2902657
- DOI: 10.1002/art.27464
Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice
Abstract
Objective: To develop an in vivo imaging method to assess lymphatic draining function in the K/BxN mouse model of inflammatory arthritis.
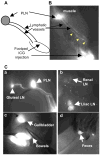
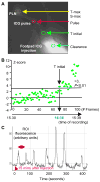
Methods: Indocyanine green, a near-infrared fluorescent dye, was injected intradermally into the footpads of wild-type mice, mouse limbs were illuminated with an 806-nm near-infrared laser, and the movement of indocyanine green from the injection site to the draining popliteal lymph node (LN) was recorded with a CCD camera. Indocyanine green near-infrared images were analyzed to obtain 5 measures of lymphatic function across time. Images of K/BxN arthritic mice and control nonarthritic littermates were obtained at 1 month of age, when acute joint inflammation commenced, and again at 3 months of age, when joint inflammation became chronic. Lymphangiogenesis in popliteal LNs was assessed by immunochemistry.
Results: Indocyanine green and its transport within lymphatic vessels were readily visualized, and quantitative measures were derived. During the acute phase of arthritis, the lymphatic vessels were dilated, with increased indocyanine green signal intensity and lymphatic pulses, and popliteal LNs became fluorescent quickly. During the chronic phase, new lymphatic vessels were present near the foot. However, the appearance of indocyanine green in lymphatic vessels was delayed. The size and area of popliteal LN lymphatic sinuses progressively increased in the K/BxN mice.
Conclusion: Our findings indicate that indocyanine green near-infrared lymphatic imaging is a valuable method for assessing the lymphatic draining function in mice with inflammatory arthritis. Indocyanine green-near-infrared imaging of K/BxN mice identified 2 distinct lymphatic phenotypes during the acute and chronic phase of inflammation. This technique can be used to assess new therapies for lymphatic disorders.
Figures



References
-
- Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–962. - PubMed
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. - PubMed
-
- Gerli R, Solito R, Weber E, Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–157. - PubMed
-
- Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. - PubMed
-
- Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH, Ahn KS, Kim J, Koh EM. Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. J Rheumatol. 2007;34:16–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

