Prognostic significance of differentially expressed miRNAs in esophageal cancer
- PMID: 20309880
- PMCID: PMC2937084
- DOI: 10.1002/ijc.25330
Prognostic significance of differentially expressed miRNAs in esophageal cancer
Abstract
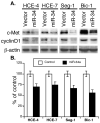
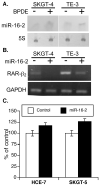
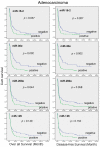
Altered microRNA (miRNA) expression has been found to promote carcinogenesis, but little is known about the role of miRNAs in esophageal cancer. In this study, we selected 10 miRNAs and analyzed their expression in 10 esophageal cancer cell lines and 158 tissue specimens using Northern blotting and in situ hybridization, respectively. We found that Let-7g, miR-21 and miR-195p were expressed in all 10 cell lines, miR-9 and miR-20a were not expressed in any of the cell lines, and miR-16-2, miR-30e, miR-34a, miR-126 and miR-200a were expressed in some of the cell lines but not others. In addition, transient transfection of miR-34a inhibited c-Met and cyclin D1 expression and esophageal cancer cell proliferation, whereas miR-16-2 suppressed RAR-β(2) expression and increased tumor cell proliferation. Furthermore, we found that miR-126 expression was associated with tumor cell dedifferentiation and lymph node metastasis, miR-16-2 was associated with lymph node metastasis, and miR-195p was associated with higher pathologic disease stages in patients with esophageal adenocarcinoma. Kaplan-Meier analysis showed that miR-16-2 expression and miR-30e expression were associated with shorter overall and disease-free survival in all esophageal cancer patients. In addition, miR-16-2, miR-30e and miR-200a expression were associated with shorter overall and disease-free survival in patients with esophageal adenocarcinoma; however, miR-16-2, miR-30e and miR-200a expression were not associated with overall or disease-free survival in squamous cell carcinoma patients. Our data indicate that further evaluation of miR-30e and miR-16-2 as prognostic biomarkers is warranted in patients with esophageal adenocarcinoma. In addition, the role of miR-34a in esophageal cancer also warrants further study.
Copyright © 2010 UICC.
Figures



References
-
- Blot W. Esophageal cancer trends and risk factors. Semin Oncol. 1994;21:403–10. - PubMed
-
- Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–29. - PubMed
-
- Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–97. - PubMed
-
- Spechler SJ. Barrett's esophagus: a molecular perspective. Curr Gastroenterol Rep. 2005;7:177–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

