Structurally and stereochemically diverse tetrahydropyran synthesis through oxidative C-H bond activation
- PMID: 20309987
- PMCID: PMC3140203
- DOI: 10.1002/anie.201000033
Structurally and stereochemically diverse tetrahydropyran synthesis through oxidative C-H bond activation
Figures
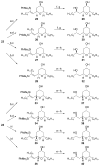

Similar articles
-
Palladium hydroxide catalyzed isomerization of primary allylic alcohols to aldehydes: application to the formal synthesis of (-)-brevisamide.Org Lett. 2011 Feb 4;13(3):382-5. doi: 10.1021/ol102658d. Epub 2010 Dec 23. Org Lett. 2011. PMID: 21182276
-
Highly stereoselective Brønsted acid catalyzed synthesis of spirooxindole pyrans.Org Lett. 2011 Jun 17;13(12):3086-9. doi: 10.1021/ol200987c. Epub 2011 May 18. Org Lett. 2011. PMID: 21591768 Free PMC article.
-
Synthesis and [4 + 2]-annulation of enantioenriched (z)-crotylsilanes: preparation of the C1-C13 fragment of bistramide A.Org Lett. 2005 Jul 21;7(15):3231-4. doi: 10.1021/ol050982i. Org Lett. 2005. PMID: 16018628
-
Recent Advances in the Synthesis of 2H-Pyrans.Molecules. 2019 Aug 9;24(16):2904. doi: 10.3390/molecules24162904. Molecules. 2019. PMID: 31405075 Free PMC article. Review.
-
Strategies for the construction of tetrahydropyran rings in the synthesis of natural products.Org Biomol Chem. 2014 Jun 7;12(21):3323-35. doi: 10.1039/c4ob00423j. Epub 2014 Apr 17. Org Biomol Chem. 2014. PMID: 24744139 Review.
Cited by
-
Quinone-Catalyzed Selective Oxidation of Organic Molecules.Angew Chem Int Ed Engl. 2015 Dec 1;54(49):14638-58. doi: 10.1002/anie.201505017. Epub 2015 Nov 4. Angew Chem Int Ed Engl. 2015. PMID: 26530485 Free PMC article. Review.
-
Asymmetric Synthesis of Functionalized Dihydro- and Tetrahydropyrans via an Organocatalytic Domino Michael-Hemiacetalization Reaction.Synthesis (Stuttg). 2014 May 1;46(9):1261-1269. doi: 10.1055/s-0033-1340826. Synthesis (Stuttg). 2014. PMID: 25284900 Free PMC article.
-
Stereoselective synthesis of spirooxindole amides through nitrile hydrozirconation.Org Lett. 2010 Nov 19;12(22):5112-5. doi: 10.1021/ol102246d. Epub 2010 Oct 20. Org Lett. 2010. PMID: 20961073 Free PMC article.
-
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone-catalyzed reactions employing MnO2 as a stoichiometric oxidant.Org Lett. 2010 Oct 15;12(20):4686-9. doi: 10.1021/ol102078v. Org Lett. 2010. PMID: 20863093 Free PMC article.
-
Synthesis of sulfur-containing heterocycles through oxidative carbon-hydrogen bond functionalization.Org Lett. 2012 Apr 6;14(7):1720-3. doi: 10.1021/ol3002877. Epub 2012 Mar 15. Org Lett. 2012. PMID: 22420412 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

