Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis
- PMID: 20329806
- PMCID: PMC3056509
- DOI: 10.2165/10898530-000000000-00000
Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis
Abstract
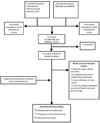
The automatic substitution of bioequivalent generics for brand-name antiepileptic drugs (AEDs) has been linked by anecdotal reports to loss of seizure control. To evaluate studies comparing brand-name and generic AEDs, and determine whether evidence exists of superiority of the brand-name version in maintaining seizure control. English-language human studies identified in searches of MEDLINE, EMBASE and International Pharmaceutical Abstracts (1984 to 2009). Randomized controlled trials (RCTs) and observational studies comparing seizure events or seizure-related outcomes between one brand-name AED and at least one alternative version produced by a distinct manufacturer. We identified 16 articles (9 RCTs, 1 prospective nonrandomized trial, 6 observational studies). We assessed characteristics of the studies and, for RCTs, extracted counts for patients whose seizures were characterized as 'controlled' and 'uncontrolled'. Seven RCTs were included in the meta-analysis. The aggregate odds ratio (n = 204) was 1.1 (95% CI 0.9, 1.2), indicating no difference in the odds of uncontrolled seizure for patients on generic medications compared with patients on brand-name medications. In contrast, the observational studies identified trends in drug or health services utilization that the authors attributed to changes in seizure control. Although most RCTs were short-term evaluations, the available evidence does not suggest an association between loss of seizure control and generic substitution of at least three types of AEDs. The observational study data may be explained by factors such as undue concern from patients or physicians about the effectiveness of generic AEDs after a recent switch. In the absence of better data, physicians may want to consider more intensive monitoring of high-risk patients taking AEDs when any switch occurs.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. There was no funding for this study.
Figures

References
-
- Strom BL. Generic drug substitution revisited. N Engl J Med. 1987 Jun 4;316(23):1456–1462. - PubMed
-
- Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006 Feb 13;166(3):332–337. - PubMed
-
- Kesselheim A, Fischer M, Avorn J. Extensions of intellectual property rights and delayed adoption of generic drugs: effects on Medicaid spending. Health Affairs. 2006;25:1637–1647. - PubMed
-
- Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry: bioavailability and bioequivalence studies for orally-administered drug products - general considerations. 2003. [Accessed 2010 Jan 21]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

