Emotion, cognition, and mental state representation in amygdala and prefrontal cortex
- PMID: 20331363
- PMCID: PMC3108339
- DOI: 10.1146/annurev.neuro.051508.135256
Emotion, cognition, and mental state representation in amygdala and prefrontal cortex
Abstract
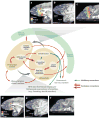
Neuroscientists have often described cognition and emotion as separable processes implemented by different regions of the brain, such as the amygdala for emotion and the prefrontal cortex for cognition. In this framework, functional interactions between the amygdala and prefrontal cortex mediate emotional influences on cognitive processes such as decision-making, as well as the cognitive regulation of emotion. However, neurons in these structures often have entangled representations, whereby single neurons encode multiple cognitive and emotional variables. Here we review studies using anatomical, lesion, and neurophysiological approaches to investigate the representation and utilization of cognitive and emotional parameters. We propose that these mental state parameters are inextricably linked and represented in dynamic neural networks composed of interconnected prefrontal and limbic brain structures. Future theoretical and experimental work is required to understand how these mental state representations form and how shifts between mental states occur, a critical feature of adaptive cognitive and emotional behavior.
Figures
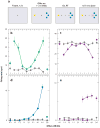
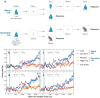
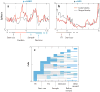

References
-
- Aggleton J, editor. The Amygdala—A Functional Analysis. Oxford: Oxford Univ. Press; 2000.
-
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) J Comp Physiol Psychol. 1981;95:961–77. - PubMed
-
- Amaral D, Price J, Pitkanen A, Carmichael S. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66.
-
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–120. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

