Interleukin-15 enhances proliferation and chemokine secretion of human follicular dendritic cells
- PMID: 20331472
- PMCID: PMC2913264
- DOI: 10.1111/j.1365-2567.2010.03252.x
Interleukin-15 enhances proliferation and chemokine secretion of human follicular dendritic cells
Abstract
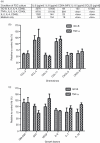
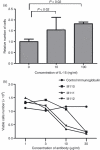
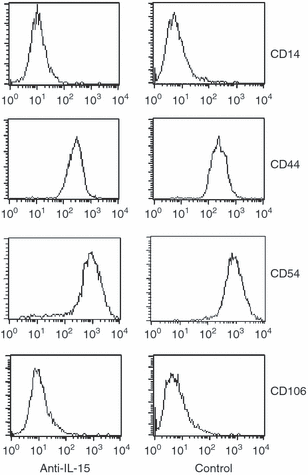
The germinal centre (GC) is a specialized microenvironment where high-affinity antibodies are produced through hypermutation and isotype switching. Follicular dendritic cells (FDCs) are the stromal cells of the GC. The timely expansion and establishment of an FDC network is essential for a protective GC reaction; however, only a few factors modulating FDC development have been recognized. In this study, we report that interleukin-15 (IL-15) enhances human primary FDC proliferation and regulates cytokine secretion. The FDCs express IL-15 receptor complexes for IL-15 signal transduction as well as for specific binding. Moreover, the secretion of chemokines CCL-2, CCL-5, CXCL-5 and CXCL-8 was reduced by blocking IL-15 signalling while the secretion of other cytokines, and the expression of CD14, CD44, CD54 (ICAM-1) and CD106 (VCAM-1) proteins remained unchanged. These results suggest that IL-15 plays a crucial role in the development of FDC networks during GC reaction, offering a new target for immune modulation.
Figures
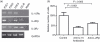

Similar articles
-
Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form.J Immunol. 2004 Dec 1;173(11):6676-83. doi: 10.4049/jimmunol.173.11.6676. J Immunol. 2004. PMID: 15557159
-
IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation.Int Immunol. 2009 Jun;21(6):745-56. doi: 10.1093/intimm/dxp041. Epub 2009 May 21. Int Immunol. 2009. PMID: 19461124 Free PMC article.
-
Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation.J Immunol. 2003 Dec 1;171(11):5975-87. doi: 10.4049/jimmunol.171.11.5975. J Immunol. 2003. PMID: 14634109
-
Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells.Semin Immunol. 2002 Aug;14(4):259-66. doi: 10.1016/s1044-5323(02)00058-1. Semin Immunol. 2002. PMID: 12163301 Review.
-
How do follicular dendritic cells interact intimately with B cells in the germinal centre?Immunology. 2005 Jan;114(1):2-10. doi: 10.1111/j.1365-2567.2004.02075.x. Immunology. 2005. PMID: 15606789 Free PMC article. Review.
Cited by
-
Expanded circulating follicular dendritic cells facilitate immune responses in chronic HBV infection.J Transl Med. 2020 Nov 7;18(1):417. doi: 10.1186/s12967-020-02584-6. J Transl Med. 2020. PMID: 33160362 Free PMC article.
-
The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use.Microbes Infect. 2012 Mar;14(3):247-61. doi: 10.1016/j.micinf.2011.10.006. Epub 2011 Oct 25. Microbes Infect. 2012. PMID: 22064066 Free PMC article. Review.
-
Importance of Crosstalk Between Chronic Lymphocytic Leukemia Cells and the Stromal Microenvironment: Direct Contact, Soluble Factors, and Extracellular Vesicles.Front Oncol. 2020 Aug 19;10:1422. doi: 10.3389/fonc.2020.01422. eCollection 2020. Front Oncol. 2020. PMID: 32974152 Free PMC article. Review.
-
Synaptic Interactions in Germinal Centers.Front Immunol. 2018 Aug 13;9:1858. doi: 10.3389/fimmu.2018.01858. eCollection 2018. Front Immunol. 2018. PMID: 30150988 Free PMC article. Review.
-
Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta.PLoS One. 2014 Feb 27;9(2):e89983. doi: 10.1371/journal.pone.0089983. eCollection 2014. PLoS One. 2014. PMID: 24587165 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

