Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes
- PMID: 20331607
- PMCID: PMC2860211
- DOI: 10.1111/j.1476-5381.2010.00667.x
Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes
Abstract
Background and purpose: Adenosine is an endogenous modulator, interacting with four G-protein coupled receptors (A(1), A(2A), A(2B) and A(3)) and acts as a potent inhibitor of inflammatory processes in several tissues. So far, the functional effects modulated by adenosine receptors on human synoviocytes have not been investigated in detail. We evaluated mRNA, the protein levels, the functional role of adenosine receptors and their pharmacological modulation in human synoviocytes.
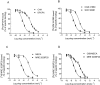
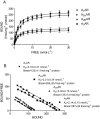
Experimental approach: mRNA, Western blotting, saturation and competition binding experiments, cyclic AMP, p38 mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-kappaB activation, tumour necrosis factor alpha (TNF-alpha) and interleukin-8 (IL-8) release were assessed in human synoviocytes isolated from patients with osteoarthritis.
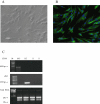
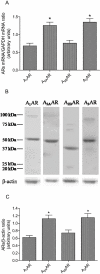
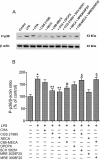
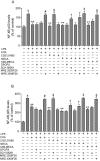
Key results: mRNA and protein for A(1), A(2A), A(2B) and A(3) adenosine receptors are expressed in human synoviocytes. Standard adenosine agonists and antagonists showed affinity values in the nanomolar range and were coupled to stimulation or inhibition of adenylyl cyclase. Activation of A(2A) and A(3) adenosine receptors inhibited p38 MAPK and NF-kappaB pathways, an effect abolished by selective adenosine antagonists. A(2A) and A(3) receptor agonists decreased TNF-alpha and IL-8 production. The phosphoinositide 3-kinase or G(s) pathways were involved in the functional responses of A(3) or A(2A) adenosine receptors. Synoviocyte A(1) and A(2B) adenosine receptors were not implicated in the inflammatory process whereas stimulation of A(2A) and A(3) adenosine receptors was closely associated with a down-regulation of the inflammatory status.
Conclusions and implications: These results indicate that A(2A) and A(3) adenosine receptors may represent a potential target in therapeutic modulation of joint inflammation.
Figures


References
-
- Abeles AM, Pillinger MH. The role of the synovial fibroblast in rheumatoid arthritis: cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis. 2006;64:20–24. - PubMed
-
- Altman R, Barkin RL. Topical therapy for osteoarthritis: clinical and pharmacologic perspectives. Postgrad Med. 2009;121:139–147. - PubMed
-
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

