Headache-type adverse effects of NO donors: vasodilation and beyond
- PMID: 20331608
- PMCID: PMC2860203
- DOI: 10.1111/j.1476-5381.2010.00643.x
Headache-type adverse effects of NO donors: vasodilation and beyond
Abstract
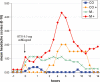
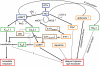
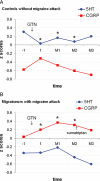
Although nitrate therapy, used in the treatment of cardiovascular disorders, is frequently associated with side-effects, mainly headaches, the summaries of product characteristics of nitrate-containing medicines do not report detailed description of headaches and even do not highlight the possibility of nitrate-induced migraine. Two different types of nitrate-induced headaches have been described: (i) immediate headaches that develop within the first hour of the application, are mild or medium severity without characteristic symptoms for migraine, and ease spontaneously; and (ii) delayed, moderate or severe migraine-type headaches (occurring mainly in subjects with personal or family history of migraine), that develop 3-6 h after the intake of nitrates, with debilitating, long-lasting symptoms including nausea, vomiting, photo- and/or phono-phobia. These two types of headaches are remarkably different, not only in their timing and symptoms, but also in the persons who are at risk. Recent studies provide evidence that the two headache types are caused by different mechanisms: immediate headaches are connected to vasodilation caused by nitric oxide (NO) release, while migraines are triggered by other actions such as the release of calcitonin gene-related peptide or glutamate, or changes in ion channel function mediated by cyclic guanosine monophosphate or S-nitrosylation. Migraines usually need anti-attack medication, such as triptans, but these drugs are contraindicated in most medical conditions that are treated using nitrates. In conclusion, these data recommend the correction of summaries of nitrate product characteristics, and also suggest a need to develop new types of anti-migraine drugs, effective in migraine attacks, that could be used in patients with risk for angina pectoris.
Figures
Similar articles
-
[Nitroglycerin-induced headaches].Orv Hetil. 2004 Nov 14;145(46):2323-8. Orv Hetil. 2004. PMID: 16106903 Hungarian.
-
The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache.Pharmacol Ther. 2008 Nov;120(2):157-71. doi: 10.1016/j.pharmthera.2008.08.003. Epub 2008 Aug 23. Pharmacol Ther. 2008. PMID: 18789357 Review.
-
Investigation of distinct molecular pathways in migraine induction using calcitonin gene-related peptide and sildenafil.Cephalalgia. 2019 Dec;39(14):1776-1788. doi: 10.1177/0333102419882474. Epub 2019 Nov 4. Cephalalgia. 2019. PMID: 31684759 Clinical Trial.
-
Investigations into the role of nitric oxide and the large intracranial arteries in migraine headache.Cephalalgia. 1997 Dec;17(8):873-95. doi: 10.1046/j.1468-2982.1997.1708873.x. Cephalalgia. 1997. PMID: 9453277 Review.
-
Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes.Cephalalgia. 2004 Jun;24(6):483-90. doi: 10.1111/j.1468-2982.2004.00691.x. Cephalalgia. 2004. PMID: 15154858
Cited by
-
Real-world safety profile of eculizumab in patients with paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, or generalized myasthenia gravis: an integrated analysis of post-marketing surveillance in Japan.Int J Hematol. 2023 Oct;118(4):419-431. doi: 10.1007/s12185-023-03630-x. Epub 2023 Jul 29. Int J Hematol. 2023. PMID: 37515657
-
Interim analysis of post-marketing surveillance of ravulizumab for paroxysmal nocturnal hemoglobinuria in Japan.Int J Hematol. 2023 Sep;118(3):311-322. doi: 10.1007/s12185-023-03625-8. Epub 2023 Jul 21. Int J Hematol. 2023. PMID: 37477863
-
Formoterol dynamically alters endocannabinoid tone in the periaqueductal gray inducing headache.J Headache Pain. 2024 Nov 19;25(1):200. doi: 10.1186/s10194-024-01907-y. J Headache Pain. 2024. PMID: 39563240 Free PMC article.
-
Headache and mechanical sensitization of human pericranial muscles after repeated intake of monosodium glutamate (MSG).J Headache Pain. 2013 Jan 24;14(1):2. doi: 10.1186/1129-2377-14-2. J Headache Pain. 2013. PMID: 23565943 Free PMC article. Clinical Trial.
-
Animal Models of Chronic Migraine: From the Bench to Therapy.Curr Pain Headache Rep. 2024 Nov;28(11):1123-1133. doi: 10.1007/s11916-024-01290-y. Epub 2024 Jul 2. Curr Pain Headache Rep. 2024. PMID: 38954246 Review.
References
-
- Abshagen U. [Controlled clinical studies of tolerance development and dosing problems in nitrate therapy] Herz. 1996;21(Suppl 1):23–30. - PubMed
-
- Abshagen U, Sporl-Radun S. First data on effects and pharmacokinetics of isosorbide-5-mononitrate in normal man. Eur J Clin Pharmacol. 1981;19:423–429. - PubMed
-
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain. 2004;110:675–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

