Glycosidated phospholipids: uncoupling of signalling pathways at the plasma membrane
- PMID: 20331609
- PMCID: PMC2860204
- DOI: 10.1111/j.1476-5381.2009.00626.x
Glycosidated phospholipids: uncoupling of signalling pathways at the plasma membrane
Abstract
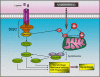
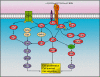
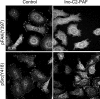
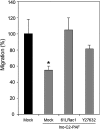
Cell expansion and metastasis are considered hallmarks of tumour progression. Therefore, efforts have been made to develop novel anti-cancer drugs that inhibit both the proliferation and the motility of tumour cells. Synthetic alkylphospholipids, compounds with aliphatic side chains that are ether linked to a glycerol backbone, are structurally derived from platelet-activating factor and represent a new class of drugs with anti-proliferative properties in tumour cells. These compounds do not interfere with the DNA or mitotic spindle apparatus of the cell. Instead, they are incorporated into cell membranes, where they accumulate and interfere with lipid metabolism and lipid-dependent signalling pathways. Recently, it has been shown that the most commonly studied alkylphospholipids inhibit proliferation by inducing apoptosis in malignant cells while leaving normal cells unaffected. This review focuses on a novel group of synthetic alkylphospholipids, the glycosidated phospholipids, which contain carbohydrates or carbohydrate-related molecules at the sn-2 position of the glycerol backbone. Members of this subfamily also exhibit anti-proliferative capacity and modulate the cell adhesion, differentiation, and migration of tumour cells. Among this group, Ino-C2-PAF shows the highest efficacy and low cytotoxicity. Apart from its anti-proliferative effect, Ino-C2-PAF strongly reduces cell motility via its inhibitory effect on the phosphorylation of the cytosolic tyrosine kinases FAK and Src. Signalling pathways under the control of the FAK/Src complex are normally required for both migration and proliferation and play a prominent role in tumour progression. We intend to highlight the potential of glycosidated phospholipids, especially Ino-C2-PAF, as a promising new group of drugs for the treatment of hyperproliferative and migration-based skin diseases.
Figures



References
-
- Andreesen R, Modolell M, Munder PG. Selective sensitivity of chronic myelogenous leukemia cell populations to alkyl-lysophospholipids. Blood. 1979;54:519–523. - PubMed
-
- Andreesen R, Modolell M, Weltzien HU, Eibl H, Common HH, Lohr GW, et al. Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res. 1978;38:3894–3899. - PubMed
-
- Ausili A, Torrecillas A, Aranda FJ, Mollinedo F, Gajate C, Corbalan-Garcia S, et al. Edelfosine is incorporated into rafts and alters their organization. J Phys Chem B. 2008;112:11643–11654. - PubMed
-
- Baburina I, Jackowski S. Apoptosis triggered by 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine is prevented by increased expression of ctp:Phosphocholine cytidylyltransferase. J Biol Chem. 1998;273:2169–2173. - PubMed
-
- Bartolmäs T, Heyn T, Mickeleit M, Fischer A, Reutter W, Danker K. Glucosamine-glycerophospholipids that activate cell-matrix adhesion and migration. J Med Chem. 2005;48:6750–6755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

