The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons
- PMID: 20331617
- PMCID: PMC2913678
- DOI: 10.1111/j.1750-3639.2010.00380.x
The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons
Abstract
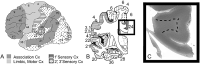
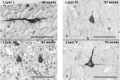
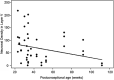
The role of the cerebral cortex in the cognitive deficits in preterm survivors is poorly understood. Periventricular leukomalacia (PVL), the key feature of encephalopathy of prematurity, is characterized by periventricular necrotic foci and diffuse gliosis in the surrounding cerebral white matter. Here, we tested the hypothesis that reductions in the density of layer I neurons and/or pyramidal neurons in layers III and/or V are associated with PVL, indicating cortical pathology potentially associated with cognitive deficits in long-term survivors. In controls (23 gestational weeks to 18 postnatal months) (n = 15), a lack of significant differences in pyramidal density among incipient Brodmann areas suggested that cytoarchitectonic differences across functional areas are not fully mature in the fetal and infant periods. There was a marked reduction (38%) in the density of layer V neurons in all areas sampled in the PVL cases (n = 17) compared to controls (n = 12) adjusted for postconceptional age at or greater than 30 weeks, when the six-layer cortex is visually distinct (P < 0.024). This may reflect a dying-back loss of somata complicating transection of layer V axons projecting through the necrosis in the underlying white matter. This study underscores the potential role of secondary cortical injury in the encephalopathy of prematurity.
Figures





Similar articles
-
Neuron deficit in the white matter and subplate in periventricular leukomalacia.Ann Neurol. 2012 Mar;71(3):397-406. doi: 10.1002/ana.22612. Ann Neurol. 2012. PMID: 22451205 Free PMC article.
-
Oxidative injury in the cerebral cortex and subplate neurons in periventricular leukomalacia.J Neuropathol Exp Neurol. 2008 Jul;67(7):677-86. doi: 10.1097/NEN.0b013e31817e5c5e. J Neuropathol Exp Neurol. 2008. PMID: 18596545 Free PMC article.
-
Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity.Pediatr Res. 2009 May;65(5):524-9. doi: 10.1203/PDR.0b013e3181998baf. Pediatr Res. 2009. PMID: 19127204 Free PMC article.
-
Progress in periventricular leukomalacia.Arch Neurol. 2008 Oct;65(10):1291-5. doi: 10.1001/archneur.65.10.1291. Arch Neurol. 2008. PMID: 18852342 Free PMC article. Review.
-
White matter injury in the preterm infant: pathology and mechanisms.Acta Neuropathol. 2017 Sep;134(3):331-349. doi: 10.1007/s00401-017-1718-6. Epub 2017 May 22. Acta Neuropathol. 2017. PMID: 28534077 Free PMC article. Review.
Cited by
-
Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization.Sci Transl Med. 2013 Jan 16;5(168):168ra7. doi: 10.1126/scitranslmed.3004669. Sci Transl Med. 2013. PMID: 23325800 Free PMC article.
-
Modeling the encephalopathy of prematurity in animals: the important role of translational research.Neurol Res Int. 2012;2012:295389. doi: 10.1155/2012/295389. Epub 2012 May 23. Neurol Res Int. 2012. PMID: 22685653 Free PMC article.
-
Neuroanatomical, sensorimotor and cognitive deficits in adult rats with white matter injury following prenatal ischemia.Brain Pathol. 2012 Jan;22(1):1-16. doi: 10.1111/j.1750-3639.2011.00504.x. Epub 2011 Aug 16. Brain Pathol. 2012. PMID: 21615591 Free PMC article.
-
Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development.J Comp Neurol. 2012 Dec 1;520(17):3912-32. doi: 10.1002/cne.23130. J Comp Neurol. 2012. PMID: 22522966 Free PMC article.
-
Stem cell-based interventions for the prevention and treatment of intraventricular haemorrhage and encephalopathy of prematurity in preterm infants.Cochrane Database Syst Rev. 2023 Feb 15;2(2):CD013201. doi: 10.1002/14651858.CD013201.pub3. Cochrane Database Syst Rev. 2023. PMID: 36790019 Free PMC article. Review.
References
-
- Anderson PJ, Doyle LW (2004) Executive functioning in school‐aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 114:50–57. - PubMed
-
- Banker BQ, Larroche JC (1962) Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol 7:386–410. - PubMed
-
- Bayless S, Stevenson J (2007) Executive functions in school‐age children born very prematurely. Early Hum Dev 83:247–254. - PubMed
-
- Benes FM, Lange N (2001) Two‐dimensional versus three‐dimensional cell counting: a practical perspective. Trends Neurosci 24:11–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

