Analysis and metabolic engineering of lipid-linked oligosaccharides in glycosylation-deficient CHO cells
- PMID: 20331963
- PMCID: PMC2887678
- DOI: 10.1016/j.bbrc.2010.03.117
Analysis and metabolic engineering of lipid-linked oligosaccharides in glycosylation-deficient CHO cells
Abstract
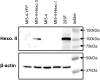
Glycosylation-deficient Chinese Hamster Ovary (CHO) cell lines can be used to expand our understanding of N-glycosylation pathways and to study Congenital Disorders of Glycosylation, diseases caused by defects in the synthesis of N-glycans. The mammalian N-glycosylation pathway involves the step-wise assembly of sugars onto a dolichol phosphate (P-Dol) carrier, forming a lipid-linked oligosaccharide (LLO), followed by the transfer of the completed oligosaccharide onto the protein of interest. In order to better understand how deficiencies in this pathway affect the availability of the completed LLO donor for use in N-glycosylation, we used a non-radioactive, HPLC-based assay to examine the intermediates in the LLO synthesis pathway for CHO-K1 cells and for three different glycosylation-deficient CHO cell lines. B4-2-1 cells, which have a mutation in the dolichol phosphate-mannose synthase (DPM2) gene, accumulated LLO with the structure Man(5)GlcNAc(2)-P-P-Dol, while MI8-5 cells, which lack glucosyltransferase I (ALG6) activity, accumulated Man(9)GlcNAc(2)-P-P-Dol. CHO-K1 and MI5-4 cells both produced primarily the complete LLO, Glc(3)Man(9)GlcNAc(2)-P-P-Dol, though the relative quantity was lower in MI5-4. MI5-4 cells have reduced hexokinase activity which could affect the availability of many of the substrates required for LLO synthesis and, consequently, impair production of the final LLO donor. Increasing hexokinase activity by overexpressing hexokinase II in MI5-4 caused a decrease in the relative quantities of the incomplete LLO intermediates from Man(5)GlcNAc(2)-PP-Dol through Glc(1)Man(9)GlcNAc(2)-PP-Dol, and an increase in the relative quantity of the final LLO donor, Glc(3)Man(9)GlcNAc(2)-P-P-Dol. This study suggests that metabolic engineering may be a useful strategy for improving LLO availability for use in N-glycosylation.
2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
None declared.
Figures



References
-
- Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. - PubMed
-
- Yurist-Doutsch S, Chaban B, VanDyke DJ, et al. Sweet to the extreme: protein glycosylation in Archaea. Mol Microbiol. 2008;68:1079–1084. - PubMed
-
- Snider MD, Rogers OC. Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell. 1984;36:753–761. - PubMed
-
- Lehle L, Strahl S, Tanner W. Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl. 2006;45:6802–6818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

