HTLV-1 p13, a small protein with a busy agenda
- PMID: 20332002
- PMCID: PMC2941701
- DOI: 10.1016/j.mam.2010.03.001
HTLV-1 p13, a small protein with a busy agenda
Abstract
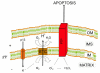
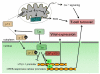
Human T-cell leukemia virus type 1 (HTLV-1) infection is characterized by life-long persistence of the virus in the host. While most infected individuals remain asymptomatic, 3-5% will eventually develop adult T-cell leukemia/lymphoma (ATLL) or tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM) after a clinical latency that can span years (TSP/HAM) to decades (ATLL). The major oncogenic determinant among HTLV-1 proteins is the Tax transactivator, which influences the expression and function of a great number of cellular proteins, drives cell proliferation, reduces cell death, and induces genetic instability. The present review is focused on the current knowledge of p13, an HTLV-1 accessory protein targeted to the inner mitochondrial membrane and, under certain conditions, to the nucleus. In mitochondria, p13 produces an inward K+current that results in an increased production of ROS by mitochondria. These effects are linked to the protein's effects on cell turnover which include activation of primary T-cells and reduced proliferation/sensitization to death of tumor cells. Recent findings suggest that in the presence of Tax, p13 is subjected to ubiquitylation and partly targeted to the nucleus. Nuclear p13 binds Tax and inhibits its transcriptional activity. These findings suggest that the protein might exert distinct functions depending on its intracellular localization and influence both the turnover of infected cells and the balance between viral latency and productive infection.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Silencers of HTLV-1 and HTLV-2: the pX-encoded latency-maintenance factors.Retrovirology. 2019 Sep 6;16(1):25. doi: 10.1186/s12977-019-0487-9. Retrovirology. 2019. PMID: 31492165 Free PMC article. Review.
-
Control of cell death pathways by HTLV-1 proteins.Front Biosci (Landmark Ed). 2009 Jan 1;14(9):3338-51. doi: 10.2741/3456. Front Biosci (Landmark Ed). 2009. PMID: 19273278 Review.
-
HSP90 protects the human T-cell leukemia virus type 1 (HTLV-1) tax oncoprotein from proteasomal degradation to support NF-κB activation and HTLV-1 replication.J Virol. 2013 Dec;87(24):13640-54. doi: 10.1128/JVI.02006-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109220 Free PMC article.
-
Target epitopes of HTLV-1 recognized by class I MHC-restricted cytotoxic T lymphocytes in patients with myelopathy and spastic paraparesis and infected patients with autoimmune disorders.J Med Virol. 2011 Mar;83(3):501-9. doi: 10.1002/jmv.21985. J Med Virol. 2011. PMID: 21264872
-
Interaction of HTLV-1 Tax protein with calreticulin: implications for Tax nuclear export and secretion.Biomed Pharmacother. 2007 May;61(4):194-200. doi: 10.1016/j.biopha.2007.02.005. Epub 2007 Mar 9. Biomed Pharmacother. 2007. PMID: 17395420 Free PMC article.
Cited by
-
Glucose Metabolism and Oxygen Availability Govern Reactivation of the Latent Human Retrovirus HTLV-1.Cell Chem Biol. 2017 Nov 16;24(11):1377-1387.e3. doi: 10.1016/j.chembiol.2017.08.016. Epub 2017 Sep 28. Cell Chem Biol. 2017. PMID: 28965728 Free PMC article.
-
HTLV-1 p13 Protein Hijacks Macrophage Polarization and Promotes T-Cell Recruitment.Viruses. 2025 Mar 26;17(4):471. doi: 10.3390/v17040471. Viruses. 2025. PMID: 40284913 Free PMC article.
-
Functional implications of mitochondrial reactive oxygen species generated by oncogenic viruses.Front Biol (Beijing). 2014 Dec;9(6):423-436. doi: 10.1007/s11515-014-1332-0. Front Biol (Beijing). 2014. PMID: 25580106 Free PMC article.
-
Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis.Front Microbiol. 2012 Dec 11;3:400. doi: 10.3389/fmicb.2012.00400. eCollection 2012. Front Microbiol. 2012. PMID: 23248621 Free PMC article.
-
Transcriptional and Epigenetic Regulatory Mechanisms Affecting HTLV-1 Provirus.Viruses. 2016 Jun 16;8(6):171. doi: 10.3390/v8060171. Viruses. 2016. PMID: 27322309 Free PMC article. Review.
References
-
- Ahsan MK, Masutani H, Yamaguchi Y, Kim YC, Nosaka K, Matsuoka M, Nishinaka Y, Maeda M, Yodoi J. Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene. 2006;25:2181–2191. - PubMed
-
- Ciminale V, Zotti L, D'Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999. pp. 4505–4514. - PubMed
-
- D'Agostino DM, Ranzato L, Arrigoni G, Cavallari I, Belleudi F, Torrisi MR, Silic-Benussi M, Ferro T, Petronilli V, Marin O, et al. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J Biol Chem. 2002;277:34424–34433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

