ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells
- PMID: 20332114
- PMCID: PMC3705932
- DOI: 10.1242/jcs.050906
ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells
Abstract
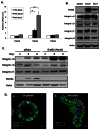
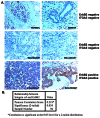
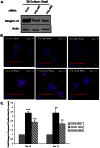
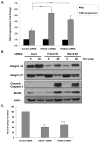
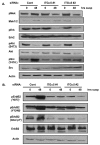
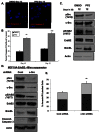
ErbB2, a receptor tyrosine kinase highly expressed in many tumors, is known to inhibit apoptotic signals. Overexpression of ErbB2 causes anoikis resistance that contributes to luminal filling in three-dimensional mammary epithelial acinar structures in vitro. Given that integrins and growth factor receptors are highly interdependent for function, we examined the role of integrin subunits in ErbB2-mediated survival signaling. Here, we show that MCF-10A cells overexpressing ErbB2 upregulate integrin alpha5 via the MAP-kinase pathway in three-dimensional acini and found elevated integrin alpha5 levels associated with ErbB2 status in human breast cancer. Integrin alpha5 is required for ErbB2-mediated anoikis resistance and for optimal ErbB2 signaling to the Mek-Erk-Bim axis as depletion of integrin alpha5 reverses anoikis resistance and Bim inhibition. Integrin alpha5 is required for full activation of ErbB2 tyrosine phosphorylation on Y877 and ErbB2 phosphorylation is associated with increased activity of Src in the absence of adhesion. Indeed, we show that blocking elevated Src activity during cell detachment reverses ErbB2-mediated survival and Bim repression. Thus, integrin alpha5 serves as a key mediator of Src and ErbB2-survival signaling in low adhesion states, which are necessary to block the pro-anoikis mediator Bim, and we suggest that this pathway represents a potential novel therapeutic target in ErbB2-positive tumors.
Figures

Similar articles
-
Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis.Mol Biol Cell. 2010 Nov 15;21(22):3829-37. doi: 10.1091/mbc.E10-04-0353. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861305 Free PMC article.
-
Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes.Mol Cell Biol. 2005 Jun;25(11):4591-601. doi: 10.1128/MCB.25.11.4591-4601.2005. Mol Cell Biol. 2005. PMID: 15899862 Free PMC article.
-
The oncogene HER2/neu (ERBB2) requires the hypoxia-inducible factor HIF-1 for mammary tumor growth and anoikis resistance.J Biol Chem. 2013 May 31;288(22):15865-77. doi: 10.1074/jbc.M112.426999. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585570 Free PMC article.
-
Anoikis mediators in oral squamous cell carcinoma.Oral Dis. 2011 May;17(4):355-61. doi: 10.1111/j.1601-0825.2010.01763.x. Epub 2010 Nov 29. Oral Dis. 2011. PMID: 21114588 Free PMC article. Review.
-
The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors.Int J Cancer. 2011 Feb 15;128(4):743-52. doi: 10.1002/ijc.25725. Epub 2010 Dec 7. Int J Cancer. 2011. PMID: 20949625 Free PMC article. Review.
Cited by
-
Caspase control: protagonists of cancer cell apoptosis.Exp Oncol. 2012 Oct;34(3):165-75. Exp Oncol. 2012. PMID: 23070001 Free PMC article. Review.
-
O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway.Oncogene. 2017 Jan 26;36(4):559-569. doi: 10.1038/onc.2016.228. Epub 2016 Jun 27. Oncogene. 2017. PMID: 27345396 Free PMC article.
-
ErbB2 stabilizes epidermal growth factor receptor (EGFR) expression via Erk and Sprouty2 in extracellular matrix-detached cells.J Biol Chem. 2011 Jan 7;286(1):79-90. doi: 10.1074/jbc.M110.169821. Epub 2010 Oct 18. J Biol Chem. 2011. PMID: 20956544 Free PMC article.
-
A Three-Dimensional In Vitro Coculture Model to Quantify Breast Epithelial Cell Adhesion to Endothelial Cells.Tissue Eng Part C Methods. 2019 Oct;25(10):609-618. doi: 10.1089/ten.TEC.2019.0122. Epub 2019 Sep 26. Tissue Eng Part C Methods. 2019. PMID: 31441384 Free PMC article.
-
Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk.Front Physiol. 2019 Jan 23;9:1942. doi: 10.3389/fphys.2018.01942. eCollection 2018. Front Physiol. 2019. PMID: 30728783 Free PMC article. Review.
References
-
- Aranda V., Haire T., Nolan M. E., Calarco J. P., Rosenberg A. Z., Fawcett J. P., Pawson T., Muthuswamy S. K. (2006). Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat. Cell. Biol. 8, 1235-1245 - PubMed
-
- Baron V., Schwartz M. (2000). Cell adhesion regulates ubiquitin-mediated degradation of the platelet-derived growth factor receptor beta. J. Biol. Chem. 275, 39318-39323 - PubMed
-
- Bromann P. A., Korkaya H., Courtneidge S. A. (2004). The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23, 7957-7968 - PubMed
-
- Comoglio P. M., Boccaccio C., Trusolino L. (2003). Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15, 565-571 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

