Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis
- PMID: 20332149
- PMCID: PMC2847467
- DOI: 10.1242/dev.043190
Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis
Abstract
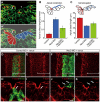
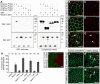
Neural tube formation is one of the most dynamic morphogenetic processes of vertebrate development. However, the molecules regulating its initiation are mostly unknown. Here, we demonstrated that nectin-2, an immunoglobulin-like cell adhesion molecule, is involved in the neurulation of Xenopus embryos in cooperation with N-cadherin. First, we found that, at the beginning of neurulation, nectin-2 was strongly expressed in the superficial cells of neuroepithelium. The knockdown of nectin-2 impaired neural fold formation by attenuating F-actin accumulation and apical constriction, a cell-shape change that is required for neural tube folding. Conversely, the overexpression of nectin-2 in non-neural ectoderm induced ectopic apical constrictions with accumulated F-actin. However, experiments with domain-deleted nectin-2 revealed that the intracellular afadin-binding motif, which links nectin-2 and F-actin, was not required for the generation of the ectopic apical constriction. Furthermore, we found that nectin-2 physically interacts with N-cadherin through extracellular domains, and they cooperatively enhanced apical constriction by driving the accumulation of F-actin at the apical cell surface. Interestingly, the accumulation of N-cadherin at the apical surface of neuroepithelium was dependent on the presence of nectin-2, but that of nectin-2 was not affected by depletion of N-cadherin. We propose a novel mechanism of neural tube morphogenesis regulated by the two types of cell adhesion molecules.
Figures





References
-
- Barrett K., Leptin M., Settleman J. (1997). The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905-915 - PubMed
-
- Bouchard M. J., Dong Y., McDermott B. M., Jr, Lam D. H., Brown K. R., Shelanski M., Bellve A. R., Racaniello V. R. (2000). Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol. Cell. Biol. 20, 2865-2873 - PMC - PubMed
-
- Burnside B. (1971). Microtubules and microfilaments in newt neuralation. Dev. Biol. 26, 416-441 - PubMed
-
- Chung H. A., Hyodo-Miura J., Nagamune T., Ueno N. (2005). FGF signal regulates gastrulation cell movements and morphology through its target NRH. Dev. Biol. 282, 95-110 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

